Expanded Reach For Global Clinical Trials
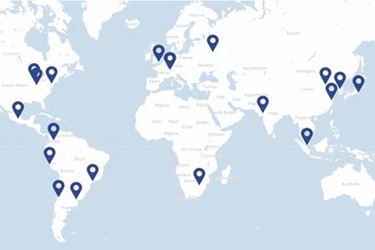
Clinical trials increasingly include patient populations that span the globe. To meet the needs of trial sponsors, Fisher Clinical Services continues to expand its global network by adding new facilities in emerging markets and enhancing capacity and services at existing sites.
During the 2014 - 2016 purpose-built, state-of-the-art cGMP facilities were opened in Suzhou, China, Singapore and Seoul, South Korea. Most recently the breadth of service offering has expanded, with Suzhou China providing new services including primary packaging, a high potent compound site, over encapsulation capabilities and bonded/non-bonded services. The recently expanded Japan facility now offers labeling for clinical trial supplies for the Asia Pacific region. Additionally, in 2017, Fisher Clinical Services Russia and South Africa facilities secured manufacturing wholesale licences, enabling secondary packaging and labeling services in addition to existing storage and distribution services offerings.
Get unlimited access to:
Enter your credentials below to log in. Not yet a member of Clinical Leader? Subscribe today.
