Rare Disease Clinical Development: Novel Approaches To Overcoming Operational Challenges

Rare diseases are serious, chronic, progressive conditions that can be disabling and/or life threatening. Most remain perplexing to medical science due to their complexity, diversity, and low prevalence. Somewhere between 7,000 and 8,000 different rare diseases have been identified and collectively, represent a significant burden on humankind as they affect an estimated 400 million people, or one out of every 10 people on the planet.
Eight out of ten rare diseases are genetic in origin, and half of all rare disease patients diagnosed are children. Today, 95 percent of all rare diseases lack a treatment approved by the US Food and Drug Administration (FDA), leaving most patients and their families with devastating consequences and little hope of a cure.
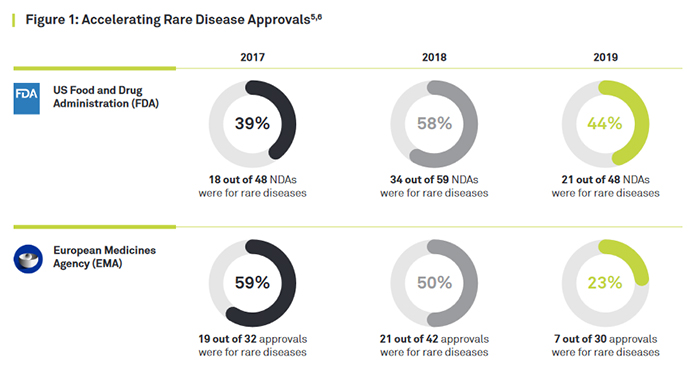
Consequently, the pharmaceutical industry is increasing its focus on developing novel therapies for many rare diseases, and approvals in the US and Europe are accelerating. In the US 21 of the 48 new approvals (44 percent) in 2019 were for rare disease indications and for Europe 21 out of 42 approvals in 2018 (See Figure 1). There are new technologies and methodologies capable of changing the game in rare disease clinical development, starting with biomarker discovery all the way through to regulatory submission.
The complexities of clinical trials in rare diseases present obstacles that slow the path to approval and add considerably to drug development costs. Getting the development plan right from the beginning of the trial is critical. The following paper reviews the challenges that biopharmaceutical sponsors face and presents a variety of solutions capable of streamlining the process to speed delivery of rare disease treatments to the market.
Get unlimited access to:
Enter your credentials below to log in. Not yet a member of Clinical Leader? Subscribe today.
