RTSM And EDC: The Unified Experience
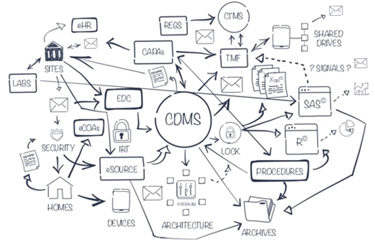
The familiar methodological framework that supports the planning, conduct and evaluation of clinical trials is changing—again. Just as manual methods for randomization, data collection, product dispensing and other core elements were replaced by electronic applications, those best-in-breed products designed to perform a single function are being replaced by integrated product suites that are up and running 24 hours a day, seven days a week, with intuitive, easy-to-follow instructions for investigators, site monitors, and users. These solutions aim to provide unified solutions with a single user interface, automatic data interchange, more reliable, robust security measures and other features that improve efficiency and quality at a lower overall cost.
Get unlimited access to:
Enter your credentials below to log in. Not yet a member of Clinical Leader? Subscribe today.
