Intent To Treat Enrichment: A Solution For Better Clinical Trials Of Precision Cancer Medicines
Eric Lynam, Executive Director and Matthew Wiener, Founder and Chief Operations Officer, Pharmatech Inc.
Restless Elephants in the Room
It is not difficult to get people who are involved in cancer clinical trials, drug development, or patient care to admit there are major problems with the way cancer research is conducted in the US. Although most patients have a positive view of clinical trials, participation remains below 5% in adults and may actually be decreasing as clinical trials become more selective and treatments more targeted. The majority of industry sponsored cancer clinical trials do not meet enrollment timelines, which both delays and adds to the cost of approved new drugs (Figure 1).
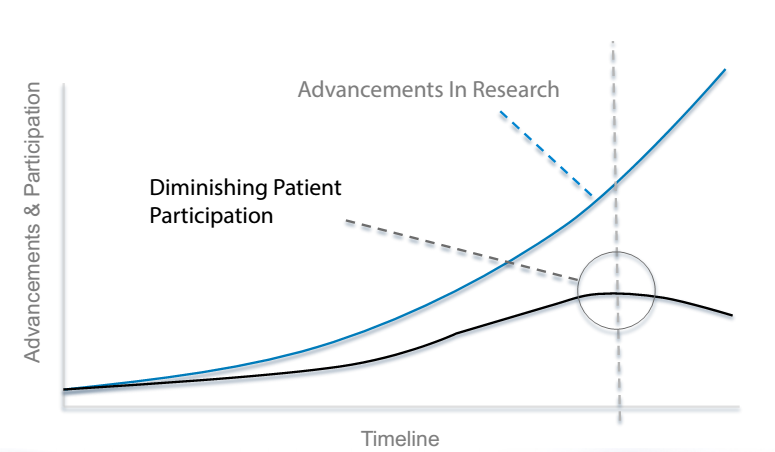
Figure 1. Critical Divergence Between Cancer Research Advances and Patient Participation
The investigators themselves face a slew of issues, including clinical and administrative workloads, patient feasibility for trials, reimbursement of study procedures, and opportunity cost. Despite the scientific and humanitarian benefits, physician time spent on clinical research is estimated to cost 20-30% in decreased financial productivity to the practice.
As cancer medicine increasingly targets specific molecular abnormalities, more of the anticancer drugs in clinical trials are precision medicines, intended to treat a highly selected niche population of patients. The opportunity for better treatment creates not only a proposition of needles and haystacks, but of hundreds of different kinds of needles sprinkled through thousands of haystacks. The traditional approach of using a fixed number of clinical trial centers and expecting each to enroll a patient or more per month is no longer realistic. Major progress against cancer requires us to innovate and find solutions that involve more patients in clinical trials and break up the gridlock we have accepted as a fact of life for too long.
Precision Cancer Medicine Calls for Patient Centered Research Solutions
Solving the needles and haystacks puzzle in cancer clinical research, requires methods to efficiently identify the patients who constitute the intent to treat population (i.e. meet all selection criteria), wherever they receive cancer care, and to connect them with the appropriate clinical trial opportunities within a clinically relevant time frame of a few weeks. A critically complicating factor is that in biomarker targeted trials, the essential qualifying diagnosis, the status of an actionable biomarker is usually an unknown. Several solutions are being pursued. All of them challenge the status quo of research dogma and biomarker assessment practice.
Patient centered research versus site centered. The first challenge to overcome is restriction of the potential sample size. The traditional site centered approach to clinical trials is based on the a priori selection of a relatively small number of research sites. Aside from the practical concern that it prioritizes administrative activities (e.g. site qualification, registration) before patient qualification, the site centered model offers a narrow and inefficient method of accessing rare and highly dispersed cancer patients. In fact, the site based system bypasses greater than 80% of cancer patients. Despite industry-wide efforts to improve the site centered research model, its fundamental limitations are compounded by today’s precision medicine trials (Table 1). A few well-chosen haystacks still contain very few needles.
|
Research Management Model |
Traditional Site Based |
Just In Time |
|
Site Registration - Initiation |
Selection – 1-2 months Registration – 1-6 months Initiation – 1 month |
Selection – Prequalified Registration – 1 week Initiation – 1 week |
|
Enrollment |
20-40% Non-enrolling sites First patient – weeks to months |
0-5% non-enrolling sites First patient – 1-2 weeks |
|
Enrollment Rate |
Standard by trial |
Accelerated – 1.3-2 fold |
|
Economic |
Sunk costs of non-enrolling sites Project Management & Monitoring Wasted test article and supplies |
95-100% of sites enroll immediately upon initiation |
Table 1. Performance Comparison: Traditional versus Just-In-Time Trial Management
In contrast, patient centered research methods identify patients across many research practices, then deliver clinical trials to meet individual patients’ immediate treatment needs. In the patient centered model, clinical trial opportunities are integrated with cancer patient care and treatment selection practices. One promising approach to this is based on the just-in-time (JIT) manufacturing concept.1 JIT research methodology prioritizes the identification of candidate patients among large investigator networks, then delivering specific trials to centers with individuals who qualify and wish to participate. Accelerated trial start up enables investigators to select the best clinical trial options ‘on demand’ for their patients’ treatment needs. By using a broader detection array, JIT is capable of identifying more research-qualified patients over a larger segment of the cancer care system than can be achieved with fixed numbers and locations of research sites. JIT research accelerates clinical trial enrollment and eliminate many of the sunk costs of nonperforming research sites.2
Multiplex molecular diagnostics versus fishing for single biomarkers. The era of precision cancer medicine is driven by the convergence of:
- a deeper understanding of the molecular biology of cancer
- the ability to routinely assess the molecular abnormalities driving individual cancers
- a growing pharmacopeia of precision cancer medicines to treat specific, actionable biomarkers.
In a world of biomarker targeted therapeutics, the fundamental inefficiencies in trial management as usual are compounded by the highly selective, niche indications sponsors pursue for FDA approval.
The current research paradigm for molecular targeted agents in clinical trial has largely focused on matching one biomarker, one patient, and one drug at a time. From the patient perspective, this is a challenging proposition, because biomarker testing usually requires the patient to consent for trial participation then delay treatment for several weeks to await results. In many cases the biomarker of interest is present in less than 10% of patients, so the likelihood of expressing a given biomarker is often a long shot. Therefore, individually or sequentially testing for biomarkers in the context of fixed trial selection requirements is slow, inefficient and of little clinical value to most patients.
Cancer researchers and trial sponsors recognize the limitations of the single test-to-trial approach. Building on the foundations laid by the Human Genome Project, ExpO, and the Cancer Genome Atlas, cancer is now more accurately viewed as a diverse set of diseases, driven by molecular abnormalities. As the road map of cancer becomes clearer, individual molecular abnormalities have merged into interrelated biochemical pathways that drive cancer progression. Clinically, understanding the full extent of mutations present in a cancer patient should suggest the most relevant treatments based on pathway inhibiting drugs. This logic is borne out by the Bisgrove study, which demonstrated that cancer patients treated according to their molecular profiles experienced clinical benefit of 26% compared to their own prior therapy, using commercially available drugs in a more targeted way. 3 Other studies have given similar results, demonstrating that molecular profile guided treatments consistently deliver better clinical outcomes than standard chemo- or radiotherapy.4,5
Integrating Molecular Diagnostics in a Patient Centered Model
The value of molecular diagnostics to identify clinically actionable biomarkers increases with each new precision drug approval. Comprehensive panel testing can be used to simultaneously identify the biomarkers with approved precision medicines as well as the biomarkers with drugs in development. Yet today, although commercially available and covered by many insurers, comprehensive molecular testing is not standard practice for most cancer patients. Until this changes, research based solutions are needed to leverage molecular diagnostic technology for better patient outcomes in both the clinical and clinical trial settings.
An ever increasing number of strategies to integrate molecular diagnostics with precision medicine trials more effectively are being pursued by pharmaceutical companies, academic alliances, and research organizations (Table 2).
|
Precision Medicine Trial Programs |
|||
|
Program |
Sponsor
|
Investigators
|
Trials Offered
|
|
Access PPM
|
Pharmatech |
Any -Open source network |
Any -Open source sponsors |
|
Signature Trial http://www.signaturetrial.com/
|
Novartis |
By trial registration |
Big Ten University Cancer Centers |
|
Big Ten Cancer Research Consortium
|
Big Ten University Cancer Centers |
6 Novartis trials (currently) |
Any -Open source sponsors |
|
Lung-MAP
|
NCI, NIH Foundation, Friends for Cancer Research, Pharma (5) |
SWOG |
Single protocol, multi drug for squamous cell lung cancer |
|
M-PACT http://clinicalstudies.info.nih.gov/cgi/
|
NCI |
NCI research centers |
Single protocol, multi drug (6) for solid tumores |
|
ORIEN
|
Moffit Cancer Center The Ohio State University Comprehensive Cancer Center |
Selected partnerships |
Single protocol, Total Cancer Care ® |
Table 2. Precision Medicine Clinical Trial Programs: 2014
The majority of these initiatives have a specific focus (e.g. a single disease indication, a single trial sponsor, an alliance of institutions), while only one is designed as an open resource for all trial sponsors, investigators and cancer patients. Each solution has unique advantages for its stakeholders, but they all share one transformative quality. They combine molecular diagnostic results with patient clinical case and treatment data to enrich the intent to treat population of biomarker targeted clinical trials. Together, the strategies of larger population sampling and prospective enrichment are the keys to a more efficient clinical trial model for rare and selective treatment indications.
Summary
Like the emerging roadmap of cancer biology, there are many pathways on the road to more effective cancer treatment through precision medicine. As the technology for molecular diagnosis of the specific abnormalities and molecular pathways driving cancer progression are integrated into clinical practice, our ability to identify and match patients with the most appropriate precision medicines and clinical trials is increasing. The prospective enrichment of intent to treat patient populations, by expanded panel or comprehensive molecular profiling, is a realistic solution to the haystacks and needles conundrum posed by precision medicine. New research based approaches are being tested, which challenge the traditional site centric view and harness the collective power of investigator networks to more efficiently connect cancer patients with research based treatment opportunities. Advancements in precision medicine are a significant part of the modern cancer treatment landscape. Together with other transformative developments in immunotherapy, molecular big data, clinical trial design, integration of precision and empirical treatment algorithms, and advancements in radiographic technology, the next 5 years should bring us much closer to realizing the full vision of personalized, precision cancer medicine.
References
- MB Wiener. Oncology drug development: Finding the right patients to complete your clinical trials. 20/20 Pharma. Q2 2011.
- EB Lynam, J Leaw, MB Wiener. A patient-focused solution for enrolling clinical trials in rare and selective cancer indications : A landscape of haystacks and needles. Drug Information Journal. 2012; 46 (4) 472-478.
- Von Hoff DD, Stephenson Jr JJ, Rosen PJ, Loesch DM, Borad MJ, et.al. Pilot Study Using Molecular Profiling of Patients' Tumors to Find Potential Targets and Select Treatments for Their Refractory Cancers. J. Clin. Oncol. 2010; 28 (33) 4877-4883.
- http://www.ispy2trial.org/about/i-spy-2-trial, accessed September 29, 2014.
- Jameson GS, Petricoin E, Sachdev JC, Liotta LA, Loesch DM, et. al. A pilot study utilizing molecular profiling to find potential targets and select individualized treatments for patients with metastatic breast cancer. J. Clin. Oncol. 31, 2013 (suppl; abstr TPS11123).
