Quality Of Outsourced Trials: Why Do Disconnects Still Exist Between Sponsors And CROs

By Steven B. Whittaker, Executive Director, Avoca Quality Consortium
The requirement for quality during the conduct of clinical trials is an absolute; a given that few in the pharmaceutical, biotech, and CRO industries would debate. Yet longitudinal survey data collected by The Avoca Group highlight that disconnects - the definitions of what constitutes quality, alignment on required levels of quality, and success at delivering to these expectations - continue to exist. Business models for designing and implementing clinical trials require that multiple stakeholder organizations (e.g. pharma/biotech, CROs, co-development partners, and regulators) align on and successfully implement to mutually agreed quality expectations.
The Avoca Group launched a seminal study in 2011 focused on perceptions of quality, particularly between sponsors and CROs. High profile warning letters had heightened awareness that challenges existed. Other factors that prompted interest in doing a deep dive into quality included the increasing trend of outsourcing from sponsors to CROs, the globalization of clinical research, and the increased complexity of clinical trials.
The survey results were both interesting and disturbing, highlighting a significant opportunity to engage stakeholder organizations to network more closely on clarifying expectations for quality and refining processes for the successful delivery to these expectations. Data from the 2011 survey highlight important and concerning differences between sponsors’ and CROs’ perceptions of quality delivered from the conduct of outsourced clinical programs. Most notable are the perception differences for “very satisfied” and “generally dissatisfied”, with sponsors being less satisfied overall.
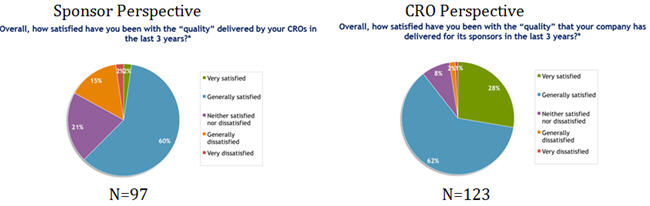
Source: 2011 Avoca Industry Survey Executive Summary
These findings were the driving factors for the creation of The Avoca Quality Consortium (AQC) in late 2011. Since that time, the consortium has focused on precompetitive opportunities to break down silos between sponsors and CROs, to clarify expectations for quality, and to develop and share leading practices for enhancing the delivery of clinical programs with higher levels of quality. Over forty member companies have collaborated on establishing and implementing leading practices. These leading practices have been shared mutually between sponsors and CROs through their partnership constructs with the intent to align on definitions of quality and to enhance the likelihood of successful delivery to expectations.
Leading practices that have been developed and utilized by the AQC underscore a range of variables that affect quality: defining quality through quality agreement templates, utilizing Quality by Design (QbD) principles, establishing top-tier clinical trial oversight guidelines and tools, creating a clinical trial metrics framework and taxonomy, implementing outcomes based and predictive quality focused metrics, and proactively identifying and managing program risks. It is incumbent upon the industry to obtain and effectively implement leading practices such as these that affect quality outcomes. And it is critical for sponsors and CROs to operationalize a more collaborative approach to quality management.
Through the AQC, there has been an opportunity to obtain longitudinal survey data from 2011 to 2015 to assess whether perceptions of quality requirements and the delivery to these expectations has improved. As depicted in the following diagram, significant disconnects remain, both in the percentage of CRO and sponsor responders being “very satisfied” and in the total number of responders being satisfied.
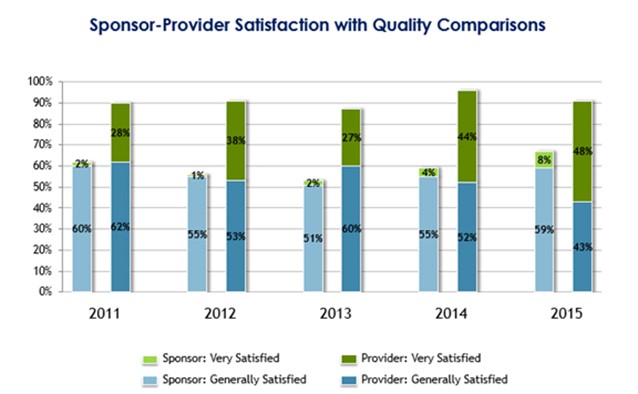
Source: 2015 Avoca Industry Survey
So while the industry has placed significant focus on quality, on the establishment of various types of strategic sourcing partnerships, and on leading practice approaches for oversight, why do such disconnects remain? Additional survey research findings and stakeholder discussions suggest the following:
- The industry has not successfully implemented sufficiently advanced enhancements in collaborative efforts between sponsors and CROs.
- Leading practices for oversight of outsourced programs, while having been developed over the past several years, have not been effectively implemented or socialized across sponsor and CRO stakeholders to truly impact long-standing cultures and processes.
- Holistic quality management systems (QMS) or frameworks have not been well defined and integrated between sponsors and CROs.
- Organizations and leaders have wavered on their stated sourcing and clinical development business models and the firmness with which they implement change initiatives.
- Significant diversity remains across sponsors in terms of desired sourcing and development models and in required standards such that CROs are challenged with effectively adapting and delivering to varied quality expectations or processes for delivery to expectations.
- Sponsors are making unilateral decisions (e.g. protocol design, site selection, regional locations for trials) that have an impact on quality and frequently are not engaging CROs in these decisions.
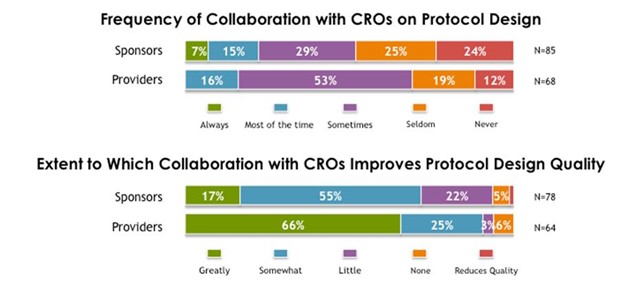
Source: 2014 Avoca Industry Survey
- Silos remain within individual sponsor and CRO organizations and across the partnerships between sponsors and CROs, whereby assumptions made by respective leaders do not reach desired clarity and alignment.
While solutions may be challenging to define and implement, it is imperative for the industry to transition beyond conversation and the development of leading practices and tools to the effective execution and socialization of leading practices within organizations and across partnerships. To achieve this will require:
- Early engagement of all relevant key stakeholders within sponsor and provider organizations in order to align on strategy as well as operational goals. A focus on aligning program objectives and definitions of success will lead to mutually agreed upon expectations and as a result, higher quality outsourced clinical trials. Early engagement should include:
- Involvement in protocol development
- Shared discussions of end goal strategies and objectives for the sponsor’s programs
- Collaboration around risk assessment and joint discussions regarding risk mitigation and management
- Clarity on constraints or challenges that may be faced by providers when implementing the program and mutually aligned approaches for solutions.
- The formal and rigorous development of integrated approaches to managing quality will provide the versatility to adapt to relevant stakeholder expectations, yet deliver absolute clarity on quality expectations. A formal and agreed upon approach to quality management will directly translate into process and measurement constructs, as well as cultural and behavioral alignment between all parties. An integrated approach should include the following:
- Quality Agreements: Clarity and documentation of mutually aligned quality expectations and definitions of quality through the use of leading practice templates. The purpose is to operationalize a conversation regarding quality expectations.
- Quality Metrics Taxonomy: A predefined approach to focus on quality outcomes of clinical programs, predictive metrics that lead to desired quality outcomes, and clarity of decision making roles and processes for utilizing quality metrics.
- Policies/Procedures: Documentation of quality expectations into operational documents that are aligned to clear business drivers.
- Vendor Management: Processes, tools, and documentation to oversee outsourced clinical trial activities.
- Training: Adequate training of staff, contractors, and vendors.
- Key Performance Indicators (KPIs) and Key Quality Indicators (KQIs): Active identification, monitoring, and reporting of performance, quality, and risk indicators.
- Clinical Risk Management: Strategies and tools to manage risk on a proactive level throughout GCP activities.
- Audit Management: Risk-based audit planning, audit management and trend analysis tools.
The foundational components of leading practices and effective quality management systems exist or are being developed through precompetitive industry engagement in consortia and other venues. The remaining hurdle of overcoming organizational and cross-partner inertia to effectively and robustly implement these foundational components into a holistic approach is large, yet it is most certainly achievable through committed joint leadership between sponsors and providers.
About The Author:
 Steven B. Whittaker is an independent consultant for the pharmaceutical, biotech, and CRO industries, providing expertise in project management, pharmaceutical development, clinical development, outsourcing strategies and execution plans. He has also consulted with clinical research sites regarding the conduct of clinical trials commensurate with sponsor expectations. He currently serves as Executive Director, Avoca Quality Consortium. His wealth of experience through years of drug development leadership roles and his established network with professionals across these industries provide a unique and valuable combination of insights for organizational leaders. Whittaker has served for thirteen consecutive years on the Advisory Board for the annual Partnerships in Clinical Trial program, chairing the board for two years. In addition, Whittaker has moderated numerous quarterly Clinical Research Consortium forums, developing strategic agendas and facilitating the exchange of leadership concepts between clinical development executives across several top-tier pharmaceutical and biotech organizations.
Steven B. Whittaker is an independent consultant for the pharmaceutical, biotech, and CRO industries, providing expertise in project management, pharmaceutical development, clinical development, outsourcing strategies and execution plans. He has also consulted with clinical research sites regarding the conduct of clinical trials commensurate with sponsor expectations. He currently serves as Executive Director, Avoca Quality Consortium. His wealth of experience through years of drug development leadership roles and his established network with professionals across these industries provide a unique and valuable combination of insights for organizational leaders. Whittaker has served for thirteen consecutive years on the Advisory Board for the annual Partnerships in Clinical Trial program, chairing the board for two years. In addition, Whittaker has moderated numerous quarterly Clinical Research Consortium forums, developing strategic agendas and facilitating the exchange of leadership concepts between clinical development executives across several top-tier pharmaceutical and biotech organizations.
Whittaker retired from Eli Lilly and Company in December 2009 where he served as Chief Operating Officer/Sr. Director of Operations and Project Management for the Cardiovascular/Acute Care Platform. He was accountable for leadership and delivery of all global phase III and IV Cardiovascular/Acute Care development portfolio projects. Prior to this, Whittaker held several leadership positions within Lilly Research Laboratories (LRL) in the areas of Regulatory Affairs (Product Registration), Discovery Research, Clinical Research, and Project Management. As the leader of the Global Clinical Research Sourcing Office, Whittaker established Lilly’s first corporate strategy and operational design for outsourcing global clinical development to CRO preferred partners. This included the selection and initial implementation of the preferred partner approach for global, full-service CRO capabilities. Whittaker also led Lilly’s Project Management Center of Excellence, establishing standards for leaders of project development.
