Risk Assessment In Clinical Trials – Well Begun Is Half Done

By Ashok Ghone, Ph.D., Vice-President, Global Services, MakroCare USA
With increased clinical R&D budgetary constraints and complexities of clinical development, risk management has become an essential and integral piece of clinical trial management to ensure good return on investment. The core of risk management is the identification and assessment of the risks in the beginning and on a continuous basis for risk-bearing activities of a clinical trial. After issuance of the guidance on Risk-Based Monitoring by FDA, the sponsors/CROs have demonstrated keen interest in adopting a systematic approach to risk assessment in clinical trials. Risk assessment is a systematic process for identifying and evaluating events that could affect the achievement of clinical study objectives. A robust risk assessment process in clinical trials forms the foundation for an effective risk management approach. It is all about taking a holistic approach to identify all potential risks and evaluate those risks to assess the probability of occurrence and impacts. Effective risk action planning involves effective risk identification and proactive root cause analysis. A right implementation of a robust risk assessment process empowers a study management team to better identify and evaluate the right risks for a clinical trial, all while maintaining the appropriate controls to ensure effective and efficient quality conduct, patient safety and regulatory compliance.
Risk Assessment Process:
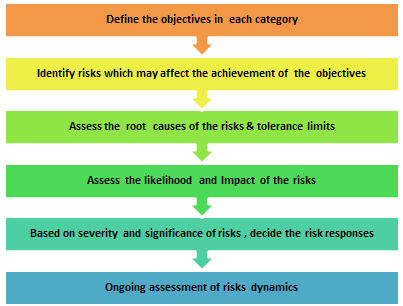
Define the objectives of each category: Identifying objectives of each category is the key in order to achieve overall goals of the study. The categories may be timelines, safety, quality and budget etc. For example, in quality category, one of the objectives would be keeping DCFs to minimal level or in timelines category, it would be meeting expected clinical trial cycle time.
Identify risks which may affect the achievements of the objectives: It is necessary to carry out detailed assessment of various internal & external processes, systems, people, vendors and technology involved in a clinical trial or a program which may pose risks to achievements of the objectives. Clear identification of risk/s which will affect the objective/s is essential for risk planning and controlling action. The examples of typical risks could be- not meeting regulatory approval timelines (timeline category) or increased in number of patients dropped-out due to AE/SAE (safety category) or number of incidences with improper ICF process/ documentation (quality) or increased in site budget (budgetary).
Assess the root causes risks and tolerance limits: Root cause analysis (RCA) is the key of risk assessment to identify the root cause, source and actual risk. Identification of right root cause facilitates effective action planning and thereby controlling or mitigation of risks. RCA also supports in improving process/system to minimize or eliminate risk completely. It is important to define tolerance or threshold for the risks as it will help in deciding trigger points to initiate corrective/mitigation measures during conduct phase. The threshold will vary study to study. For example, for patient recruitment rate, threshold could be >20% sites below expected recruitment rate or for DCFs status, it could be >25% pending DCFs for 30 days or more.
Assess the likelihood and impact of the risks: There are different matrix used to assess the likelihood and impact of the risks. These are based on 1to 3 scale or 1 to5 scale which provides a simple mechanism to increase visibility of risks and assists in management of risks. The root cause analysis helps in assessing the risk source further for its chances of occurrence (likelihood) and impact (consequence) it may have on a study. Risk delectability is another dimension which can be considered along with likelihood and impact in the risk assessment process.
Based on severity and significance of risks, decide the risk responses: The risk responses should be planned appropriately based on the risk score and risk significance. The risk responses and action planning should be done appropriately for high and extreme (critical) risks. The responses could be one of the four types – Avoid, Transfer, Accept or Mitigate. ‘Mitigate’ response is very commonly used. Mitigation actions may have one of different objectives like- eliminate the risk completely, minimize the impact of the risk, reduce the likelihood of the risk occurring, or increase the chances of the risk being detected if it should occur. Example of Mitigation response could be – selecting an investigational site having excellent patient pool but with less or no GCP experience. This risk can be mitigated by close monitoring of such site or additional training to the site
Robust risk assessment in the beginning and planning of a clinical trial help to win half the battle. However, risks are dynamics and therefore, risk assessment should also be ongoing during conduct and closing phase of a trial. There are chances that one risk may give rise to another or multiple risks or there may be change or increase in root causes of a risk. Ongoing assessment of risk helps to fine tune risk response and action planning to improve overall risk management.
The right technology can bring great efficiencies and effectiveness in risk assessment & management process. As these are dynamic processes, they require proper technology that can catch various aspects and changing scenarios of the risks to get full picture of the risks involved. As the industry is moving towards Quality Risk Management (QRM) or Risk-Based Monitoring (RBM) approach, currently the market has witnessed the customized tools for risk management catering to the need of clinical trials project/program.
Ashok Ghone, Ph.D. is Vice-President, Global Services at MakroCare USA. He has around 20 years of experience in the pharmaceutical and clinical research industry. Ashok has good knowledge and understanding of global clinical research with hands-on experience in clinical operations, project management, risk management, process development, document management, site management and patient recruitment activities. He has led various cross-functional teams successfully by providing strategic direction and guidance for accomplishment of local, regional and global projects involving early and late phase clinical studies in various therapeutic areas. Ashok has been involved in development of process, system and training related to risk-based monitoring and centralized monitoring at MakroCare, which offers these specialized services to biopharmaceutical and medical device companies to support their endeavors in implementation of the RBM, Centralized Monitoring approach.
Email: ashok.ghone@makrocare.com
