How To Measure Progress In Improving Clinical Trial Diversity
By Sara Bristol Calvert, Pharm.D., Clinical Trials Transformation Initiative (CTTI)

The last several years have included many actions and acknowledgments of the need to improve the equitable access to and diverse participation in clinical trials. The National Academies of Sciences, Engineering, and Medicine, the FDA, and the NIH have released several policies and statements that encourage diversity and inclusion in clinical trials.1,2,3 In addition, the Food and Drug Omnibus Reform Act (FDORA)4 will require the submission of diversity action plans by medical product sponsors to the FDA.
Previously, many efforts to improve clinical trial diversity included focused site-level recruitment and retention strategies. These efforts were often created for an individual clinical trial, were often not maintained when a trial closed, and may not have had lessons learned applied to new trials. Therefore, the Clinical Trials Transformation Initiative — a public-private partnership founded by Duke University and the FDA — conducted a Diversity in Clinical Trials Project focused on implementing diversity and inclusion practices at the organizational level to create sustainable changes in the design and conduct of the institution’s clinical trials.
In this article, the CTTI Diversity project’s recommendations and a maturity model are discussed. The products are intended for organizations that design and conduct clinical trials to develop or improve sustained clinical trial research infrastructure that is more responsive to the needs of historically underrepresented populations.
What Are The Benefits Of Developing Organizational-Level Diversity In Clinical Trial Strategies?
The CTTI Diversity Project included interviews with leaders who participate in decisions on resource allocation and/or who are responsible for leading the implementation of organizational-level diversity and inclusion activities. These leaders shared that organizational-level practices designed to improve equitable access and diverse participation in clinical trials led to better science, better treatments, and improved patient trust (Corneli CP&T). Enrolling participants that more closely represent those intended to use the medical product, if approved, results in more accurate and generalizable trial results by enhancing and expanding our understanding of the safety and efficacy of investigational medical products. Increasing diverse patient participation in clinical trials also can improve access to innovative and potentially life-extending or life-improving therapies, improve trust in clinical trial results, and facilitate patient uptake if the medical product is approved. Improved trust of the organization from sustained engagement and partnerships with patients and communities also leads to better recruitment and retention in the organization’s clinical trials.
What Organizational-Level Strategies Are Recommended?
Through the analysis of the interview results and a discussion of the results at a multi-sector expert meeting, recommendations with four action areas were created to advance the sustainable support of clinical trial diversity, equitable access, and inclusion: commitment, partnerships, resources, and accountability.
Commitment
Commitment includes senior leadership driving and being accountable for establishing a strong organization-wide commitment toward the implementation of clinical trial diversity strategy. The second part of commitment includes ingraining principles of diversity and inclusion within the organizational culture, overall research strategy, and during the development of all new clinical trials.
Partnerships
Partnerships include bidirectional relationships with community groups and other community representatives, engaging patients and patient groups, and collaboration with operational partners in the clinical trials ecosystem. Bidirectional community partnerships should be established and maintained between trials to inform the organization’s research priorities, the development of clinical trial diversity programs, and early community contribution during new trial development and with implementation of individual clinical trials.
Diverse individual patient and caregiver representatives and patient groups should be included in the development of clinical trial diversity programs and at all stages of medical product development. The action area of resources includes investing sufficiently and sustainably in the development and consistent deployment of strategies to ensure equitable access to and diverse participation in clinical trials in a way that sustainably extends beyond the length of individual clinical trials, programs, or grants.
Resources
In addition, dedicated personnel, at all levels of the organization, should be accountable for the design and deployment of clinical trial diversity strategy, share expertise, and facilitate strong, cross-functional coordination and collaboration across the organization.
The second part of the resources area includes investing sufficiently and sustainably in the development and consistent deployment of strategies to ensure equitable access to and diverse participation in clinical trials in a way that sustainably extends beyond the length of individual clinical trials, programs, or grants.
Accountability
Finally, accountability includes using data-driven strategies to identify the needs of diverse populations, monitoring the recruitment and retention of diverse participants in the organization’s clinical trials, and using the data collected to continuously improve clinical trial diversity programs.
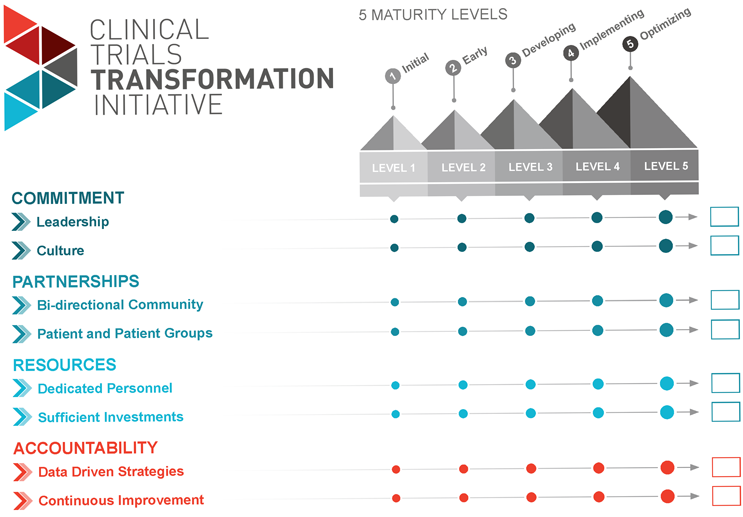
Using A Maturity Model To Develop Or Improve Clinical Trial Diversity Programs
As previously mentioned, many organizations have taken action to improve clinical equitable access and diverse participation in clinical trials. This may include study-level practices or organizational commitments. Therefore, the project team developed a tool for organizations to assess their current clinical trial diversity infrastructure and to identify a desired future state — the Diversity Maturity Model for Organizational-Level Strategies.
The Maturity Model focuses on eight elements of organizational-level infrastructure and is aligned with CTTI’s Diversity in Clinical Trials Recommendations. Each element is scored from level 1 to 5, where level 1 implies little or no coordinated organizational-level strategies, levels 2-4 imply increasingly complete and effective organizational-level strategy implementation, and level 5 describes an idealized state of complete implementation, along with continuous improvement efforts.
For example, in the development of bidirectional community partnerships, an organization at level 1 may participate in occasional community outreach for study recruitment but does not have a sustained, coordinated approach for identifying and approaching community groups or representatives. At level 3, the organization is developing a strategy for creating community partnerships and may have some community partnerships, but the partnerships and insights are not shared within the organization or reflected in the overall diversity program. At level 5, bidirectional community partnerships exist to optimize clinical trial diversity efforts and the design and planning of clinical trials, with coordination across the organization.
Organizations can choose how they use the model; some rows in the model may not apply to all organizations, and the desired future state may also vary. For example, we received feedback from an academic research institution that assessed its status for community partnerships and patient engagement and used the results to advocate for support to develop additional training for patient navigators and resources toward community outreach. A pharmaceutical company adapted the maturity model and is using it to assess each product development program within the company to determine where training, staff, or other resources are most needed as well establish reports to share progress with senior leadership.
The CTTI diversity recommendations and maturity model are applicable for organizations at various stages in their development of organization-wide strategies for improving equitable access and participation of diverse populations in clinical trials. The recommendations describe best practices, and the supporting maturity model is intended to guide organizations, including those in early stages of building organizational-level strategies, in the assessment of the current state of development and identification of their ultimate desired progress.
References:
- National Academies of Sciences. “Improving Representation in Clinical Trials and Research: Building Research Equity for Women and Underrepresented Groups.” Building Research Equity for Women and Underrepresented Groups | The National Academies Press, 17 May 2022, nap.nationalacademies.org/catalog/26479/improving-representation-in-clinical trials-and-research-building-research-equity.
- Commissioner, Office of the. “Clinical Trial Diversity.” U.S. Food and Drug Administration, FDA, www.fda.gov/consumers/minority-health-and-health-equity/clinical trial-diversity. Accessed 5 Oct. 2023.
- “NIH Policy and Guidelines on the Inclusion of Women and Minorities as Subjects in Clinical Research.” NIH, U.S. Department of Health and Human Services, grants.nih.gov/policy/inclusion/women-and-minorities/guidelines.htm. Accessed 5 Oct. 2023.
- One Hundred Seventeenth Congress of the United States of America, www.congress.gov/117/bills/hr2617/BILLS-117hr2617enr.pdf. Accessed 5 Oct. 2023.
About The Author:
 Sara Bristol Calvert, Pharm.D. is the director of projects at the Clinical Trials Transformation Initiative. Dr. Bristol Calvert provides senior operational leadership and oversight for CTTI’s project portfolio. She has worked collaboratively with multi-sector teams to create recommendations and tools in areas such as registry-based clinical trials, pregnancy testing plans, feasibility of early informed consent, using a single IRB approach, and increasing diversity in clinical trials. Prior to joining CTTI, Dr. Bristol Calvert was a project leader at the Duke Clinical Research Institute and practiced as a clinical pharmacist at the Duke Outpatient Clinic. Dr. Bristol Calvert received a Pharm.D. from the University of Pittsburgh and completed a specialty residency in primary care pharmacy practice at Duke University Medical Center and Health System.
Sara Bristol Calvert, Pharm.D. is the director of projects at the Clinical Trials Transformation Initiative. Dr. Bristol Calvert provides senior operational leadership and oversight for CTTI’s project portfolio. She has worked collaboratively with multi-sector teams to create recommendations and tools in areas such as registry-based clinical trials, pregnancy testing plans, feasibility of early informed consent, using a single IRB approach, and increasing diversity in clinical trials. Prior to joining CTTI, Dr. Bristol Calvert was a project leader at the Duke Clinical Research Institute and practiced as a clinical pharmacist at the Duke Outpatient Clinic. Dr. Bristol Calvert received a Pharm.D. from the University of Pittsburgh and completed a specialty residency in primary care pharmacy practice at Duke University Medical Center and Health System.
