One Step

Make strides with a tailored, comprehensive suite of discovery and development services to rapidly progress your project and reduce time to market.
Reduce time to market, simplify project management efforts, and keep costs down with the new Charles River One Step program. With one point of contact at one CRO partner, our clients have an accessible and knowledgeable advocate, from bench to bedside. Charles River’s One Step projects are driven by scientific excellence and leverage deep therapeutic area expertise including neuroscience, oncology, immunology, metabolic, respiratory, cardiovascular, and gastrointestinal.
Our track record of success includes over 90 nominations of small molecule compounds into clinic. Our chemistry team is cited as inventors on over 450 patents across a variety of therapeutic areas and target classes including: GPCR, kinase, enzyme, chemokine, ATPase, ion channel, monoclonal antibody, and NHR.
We have worked on over 80% of the FDA-approved drugs over the past three years and successfully deliver over 1,300 IND programs each year. Regulatory experts on One Step projects average over 500 client and agency inspections each year. Together, we can trim one full year from drug development, enabling faster therapeutic delivery to patients.
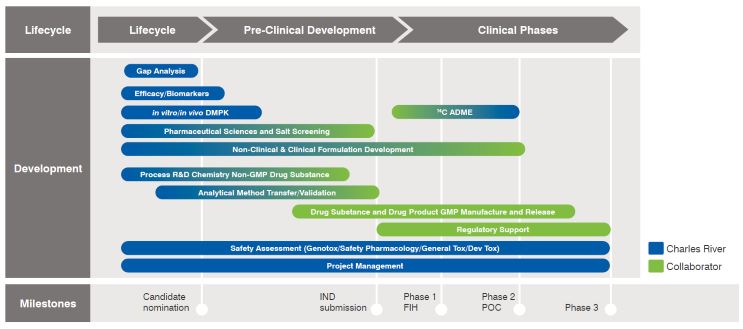
- Target and Drug Discovery
- Candidate Identification
- Pre-FIH or IND Development
- Support for Clinical Phases
Target and Drug Discovery
Charles River’s One Step offers target validation to lead optimization, bringing forward the most promising lead compounds using our biology, chemistry, DMPK, structural biology, and computer-aided drug design capabilities. In addition, we establish in vivo-in vitro correlations to predict in vivo pharmacology outcomes, conduct early in vitro safety assays to minimize risk to compound progression, and employ a translational biology approach to connect in vitro and in vivo data to clinically relevant markers.
Candidate Identification
We offer efficacy and dose-to-human projection established in relevant models, non-GLP safety studies (DRF/MTD) to provide an early indication of therapeutic index (TI), and early understanding of pharmaceutics and formulation development challenges. At this stage, we also manage process research to understand synthetic route scalability for development.
Pre-FIH or IND Development
One Step includes GLP safety studies to de-risk potential for drug-to-drug interactions and meet regulatory requirements, expertise to meet these regulatory requirements: GLP ICH S7A Core battery safety pharmacology, GLP in vitro genotoxicity studies, and GLP repeat dose toxicity studies. One Step offers formulation(s) analytical method development and validation to support in vitro and in vivo safety studies, efficiency gains in tech transfer from discovery through development and into CMC to develop drug substance and drug product.
In addition, One Step includes bioanalytical analytical methods development and validation to support GLP safety studies, and advice on regulatory strategy and program design to ensure an efficient path to FIH trials.
The One Step team coordinates activities from early discovery all the way through to GMP and drug product development. We will ensure a clear CMC strategy in place during early lead optimization, including development chemistry carried out at the right time to support the most rapid progress into clinical enabling studies and seamless, comprehensive methods transfer into a trusted CDMO.
Support for Clinical Phases
Every One Step program includes holistic scientific advice, regulatory support, and recommendations in addition to multi- disciplinary, highly interactive project teams, and CMC know-how in one team.
Also included are GLP ADME studies to understand in vivo metabolism and routes of excretion, and reproductive, chronic toxicity and carcinogenicity studies to support longer duration clinical trials to market.