Understanding Human Behavior To Help Improve Patient Adherence
By Claire Everitt, device design & engineering team lead, R&D, Pfizer, and industry project lead, BEAMER, and Aad R. Liefveld, project member, BEAMER

Adherence is essential to maximize patients´ benefit from treatment and therapy, a key factor for subsequent health outcomes. Lack of adherence is associated with poor patient outcomes and increasing healthcare costs. Yearly, 200,000 premature deaths in the EU1 are related to non-adherence, which causes personal suffering and a significant cost burden on healthcare systems2. In the U.S., non-adherence can account for around 125,000 premature deaths, up to 50% of treatment failures, and up to 25% of hospitalizations each year3. The annual costs in Europe of avoidable hospitalizations, emergency care, and adult outpatient visits are assessed at 125 billion euros1 and similar figures exist for other regions in the world.
Mounting evidence shows that 25% of patients are non-adherent to prevention activities and disease management activities including medication intake, use of medical devices, appointment scheduling, screening, exercise, and dietary changes4. Focusing on treatment, estimates show that nearly 50% of patients do not succeed in following recommendations5. More specifically, when regimens are complex and/or require changes to existing habits or lifestyle, non-adherence can be as high as 70%6. Medication and medical device non-adherence is a significant barrier to managing chronic conditions effectively, leading to poorer health outcomes among patients, higher rates of hospitalization, and increased mortality. This contributes to financial burdens on healthcare systems and overall societal costs7. It also impacts the assessment of drug and medical device effectiveness in clinical studies.
Improving adherence is a way to help ensure the best possible patient outcome and to make healthcare and societal costs more viable while maximizing the patient’s experience.
A Lot Has Improved, But Not Enough
A patient’s behavior is the most critical factor influencing the outcome of a treatment. As former U.S. Surgeon General C. Everett Koop said in 1985, “Drugs don’t work in patients who don’t take them.”
Over the years, the healthcare industry has worked to enhance adherence by improving patient experience, creating partnerships between healthcare professionals and patients based on shared decision-making. Drug development has become more patient-centric, leading to less burdensome clinical studies and clinical care treatments. Patient information is now more focused on patient needs, leading to a better understanding of condition and treatment.
However, statistics show this has not impacted adherence rates significantly. To address this the Innovative Medicines Initiative 2 Joint Undertaking (IMI2), a public-private partnership between the European Union (EU) and the European Federation of Pharmaceutical Industries and Associations (EFPIA) under the Horizon 2020 program, and IMI associated partner Link2Trials approved the BEAMER8 project (grant agreement number 101034369) with the aim of improving patient support and treatment adherence.
Joining Forces To Improve Adherence
BEAMER stands for BEhavioral and Adherence Model for improving quality, health outcomes and cost-Effectiveness of healthcaRe. Its aim is to provide a better understanding of the psychological factors that affect patient adherence behavior to enable the healthcare industry to design and deliver more targeted and relevant support. BEAMER will generate an open-source model, grounded in behavioral science, with the significant factors affecting adherence. The model will identify subpopulations based on patient needs, which will provide a foundation for future real-world solutions to improve adherence behavior, enabling support solutions for the right patients at the right time.
To achieve this, 27 key stakeholders (Figure 1) are collaborating across the healthcare chain, including universities, healthcare providers and hospitals, patient organizations, research and technology companies, healthcare related nonprofit organizations, and global pharmaceutical companies, to provide a validated model ready for adoption by its primary stakeholders and beneficiaries in three years’ time.
Targeting Real-World Adherence Improvements
The longer-term goal of BEAMER is to achieve real-world adherence improvements. For that to happen, it is essential that stakeholders select and adopt it as the foundation for solutions that bring real value to their daily lives. Consequently, the model must be more than a comprehensive, theoretical concept. It also needs to be simple, pragmatic, proven to be effective, and based on factors that we can influence.
Behavior Based And Disease-agnostic
Although BEAMER is not the only initiative, past or present, seeking to better understand and improve adherence, its strength is in its focus on the underlying behavioral factors affecting adherence which, unlike age or education level, will change from person to person and throughout the patient’s health and wellness journey. These behavioral factors, such as a person’s health beliefs and their need for control or trust, are not associated with any single disease state but are applicable across all conditions. Consequently, BEAMER can develop a disease-agnostic baseline model grounded in behavioral science and focused on significant factors affecting adherence behavior to identify the support needs of individual patients.
Actionable Factors
The purpose of BEAMER is not only to generate a better understanding of adherence behavior but also to effectively improve adherence behavior during treatment. This requires the factors of the model to be not only significant but also actionable. Previous research has shown that more quantitative factors, such as education level, income, or age, are significant but not easily modified. An advantage of using behavioral factors is that they are dynamic which, while challenging, means that they can be changed with the right support. Patient motivation is relatively underrepresented in literature but is critical to adherence9 and can be addressed throughout the patient journey.
Pragmatic, Simple, And Effective
The BEAMER model aims to balance simplicity with personalization to make it easy to use but effective in guiding improvements to adherence. To ensure widespread use of the model and consequent benefits to patients, it is essential to minimize the patient burden, which in turn drives the need to identify the factors of most significance for the implementation. Given the impact on patients, a simple and pragmatic approach also has the benefit that it can be implemented to improve adherence sooner than later. At a future stage, by applying the experience of using the model in the real world, we foresee iterations to improve and fine-tune the model.
Validated
Validation is essential for a model to be accepted, implemented, and used as the foundation for tangible solutions. Validation is planned through clinical studies with a total of 18,000 patients in six different treatment areas: cardiovascular diseases, oncology, immunology, neurology, endocrinology, and rare diseases. This aims to demonstrate that the model provides effective and disease-agnostic understanding of patients in a format that is relevant to all stakeholders.
For Clinical Care And Clinical Research
A clinical research study is a much more controlled environment than treatment in clinical care, but there are significant similarities. In both, human beings, responding and behaving according to the factors that influence their status quo, are involved. BEAMER originated from a real-life issue in clinical care but, due to its behavioral approach, it is fully compatible with the clinical research environment.
Non-adherence is considered a greater issue during clinical care than in clinical studies; however, genuine issues also exist in clinical studies. After decades of improving clinical studies, the early dropout rate, across all conditions, is still substantial: up to 30%10. And almost 70% of the protocol deviations are potentially linked to non-adherence11. Non-adherence therefore has a major effect on timelines, costs, efficacy, and clinical data sets. Additionally, the initial stages of clinical research provide an opportunity to develop products to better meet the needs of the target population, resulting in fewer early dropouts and reduced time and cost.
A Future With BEAMER
Three years from now, the BEAMER project will deliver an environment where healthcare stakeholders can check a patient’s behavioral phenotype and the type of support needed to sustain or improve adherence behavior. Here, researchers can run simulations for specific treatments and target populations. The model and its phenotypes will be open source to provide healthcare professionals, technology companies, service providers, and the pharmaceutical industry with the necessary understanding to develop effective real-world solutions to provide personalized adherence support to patients.
The implementation of BEAMER will be scalable and useable across the healthcare chain. Combining the characteristics of the condition, the BEAMER profile or segment, and the treatment will indicate when and how each patient needs to be supported for optimal adherence.
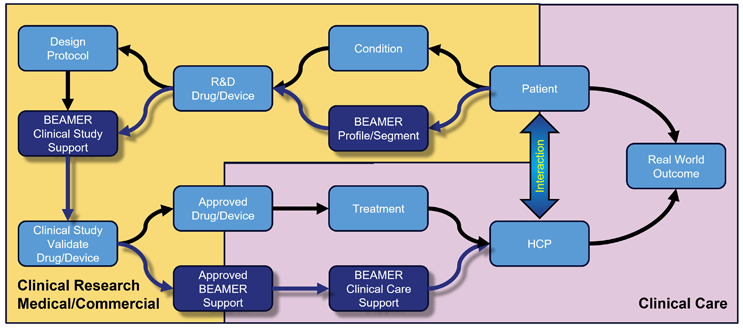
In clinical research (Figure 2), BEAMER can help target patient support to improve persistence, lower early dropout rates, limit the number of protocol deviations, reduce missing data, and may improve real-world effectiveness.
Healthcare professionals in primary care and at hospitals, interacting directly with patients, can apply BEAMER to patient support procedures, either on an individual basis or with integrated digital solutions. Medical departments at pharmaceutical companies can use BEAMER to design or commission specific patient support programs for their products and deliver both together to the market. Finally, technology companies and service providers can take the same approach and build specific digital solutions for drugs and devices that are already on the market. When done correctly, these solutions could potentially be integrated with local hospital solutions to support multi-condition and polypharmacy situations.
Why Is BEAMER Important To All Of Us?
At some point in time, we will all either be a patient or support a patient (parents, partners, children). We want to maintain our quality of life for as long as possible and better personalization enables us to receive more tailored and relevant support to achieve this.
From a broader perspective, we also need our healthcare to be efficient, effective, and sustainable as it is a key constituent in the smooth functioning of our society. However, it also needs to be financially efficient — we expect both quality and “value for money” from our healthcare services. Non-adherence to treatment not only has a direct effect for patients but also a significant negative impact on overall healthcare costs. Managing and even reducing these costs is high on the agenda of governments and payer organizations all over the world.
With BEAMER, we are generating greater insight into patients’ needs and challenges. Consequently, it will provide a foundation to help develop more effective patient support and treatment decisions that can improve the patient’s experience and better support healthcare professionals. If applied to clinical trials, it could also reduce dropout rates, costs, and timescales and provide early feedback into the development process. With broad implementation it could lower overall healthcare costs and lessen the burden that diseases and non-adherence to treatment are putting on our society.
About The Authors:
 Claire Everitt is device design & engineering team lead at Pfizer R&D UK and the industry project pead for the BEAMER project. At Pfizer, Claire is responsible for mechanical engineering and usability with a focus on human factors and adherence to treatment. Having worked in many other industries, she brings experience and expertise in the field of project management, human-centered design, and usability. Claire holds a master’s degree in engineering from the University of Southampton and a BSc in psychology from the Open University.
Claire Everitt is device design & engineering team lead at Pfizer R&D UK and the industry project pead for the BEAMER project. At Pfizer, Claire is responsible for mechanical engineering and usability with a focus on human factors and adherence to treatment. Having worked in many other industries, she brings experience and expertise in the field of project management, human-centered design, and usability. Claire holds a master’s degree in engineering from the University of Southampton and a BSc in psychology from the Open University.
 Aad R. Liefveld is a member of the BEAMER project team and a member of the advisory board at Link2Trials. He has over 30 years of management experience across multiple industries and is a strong advocate of patient centricity and patient experience. Together with Prof. Dr. Sjaak Bloem (Nyenrode Business University), Aad has developed the Adherence Risk Management Services for Link2Trials to improve patient adherence during clinical studies.
Aad R. Liefveld is a member of the BEAMER project team and a member of the advisory board at Link2Trials. He has over 30 years of management experience across multiple industries and is a strong advocate of patient centricity and patient experience. Together with Prof. Dr. Sjaak Bloem (Nyenrode Business University), Aad has developed the Adherence Risk Management Services for Link2Trials to improve patient adherence during clinical studies.
For more information, follow the BEAMER project on LinkedIn.
References:
- OECD/European Union. (2018). Health at a Glance: Europe. Paris/Brussels: OECD Publishing/European Union
- Cutler RL, Fernandez-Llimos et al. Economic impact of medication non-adherence by disease groups: a systematic review. BMJ Open 2018;8:e016982
- Kim J, Combs K, Downs J, Tillman III F. Medication Adherence: The Elephant in the Room. U.S. Pharmacist. Available at: https://www.uspharmacist.com/article/medication-adherence-the-elephant-in-the-room.
- Di-Matteo, R. (2004). Variations in patients' adherence to medical recommendations: a quantitative review of 50 years of research. MedCare, 200-209
- Anglada-Martinez, H., Riu-Viladoms, G., et al. (2015). Does mHealth increase adherence to medication? Results of a systematic review. Int J Clin Pract, 9-32. 6 Chesney, M. A., Morin, M., & Sherr, L. (2000). Adherence to HIV combination therapy. Soc Sci Med, 1599-1605 LI, B. D., Brown, W. A., & Ampil, F. L. (2000). Patient compliance is critical for equivalent clinical outcomes for breast cancer treated by breast-conservation therapy. Ann Surg, 883-889
- Nieuwlaat R, Wilczynski N, Navarro T, Hobson N, Jeffery R, Keepanasseril A, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev 2014; 20
- Cutler RL, Fernandez-Llimos F, , et al Economic impact of medication non-adherence by disease groups: a systematic review BMJ Open 2018;8:e016982
- https://www.ihi.europa.eu/news-events/newsroom/beamer-aims-boost-adherence-treatment
- Adherence to Long-Term Therapies: evidence for action. World Health Organisation 2003
- National Academy of Sciences . The Prevention and Treatment of Missing Data in Clinical Trials. Washington, D.C.: National Academies Press; 2010 https://nap.nationalacademies.org/catalog/12955/the-prevention-and-treatment-of-missing-data-in-clinical trials
- https://www.linkedin.com/pulse/protocol-deviations-tell-tale-signs-patient-during-trials-liefveld/?trackingId=tH%2BFkrY2S1GQekonW1u9OQ%3D%3D
