Expedited Study Delivery - Logistics
Source: QPS LLC
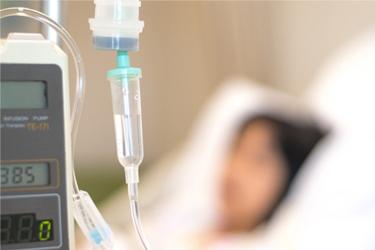
A biotech Sponsor approached QPS to conduct a challenging Phase I SAD/MAD study, involving multiple IV doses over multiple days in healthy volunteers. The design included first-in-human, safety, tolerability and PK of multiple escalating IV infusion doses. The study subjects received multiple infusions of study drug, during daytime and nightime hours, on each study day.
This case study outlnes the 3 factors that allowed QPS to deliver a complex, data-intensive early phase clinical trial, and collected approximately 1,000,000 data points in just 2 months.
access the Case Study!
Log In
Get unlimited access to:
Trend and Thought Leadership Articles

Case Studies & White Papers

Extensive Product Database

Members-Only Premium Content

Welcome Back! Please Log In to Continue.
X
Enter your credentials below to log in. Not yet a member of Clinical Leader? Subscribe today.
Subscribe to Clinical Leader
X
Subscribe to Clinical Leader
