A Better Approach To Selecting And Overseeing GCP/GLP Vendors And Processes
By Judy Carmody, Ph.D., Carmody Quality Solutions, LLC
This is the third article in a series examining strategies that allow quality groups to collaborate with good clinical practice (GCP) and good manufacturing practice (GMP) divisions to improve compliance, increase clinical study robustness, and enhance data integrity.
(GCP) and good manufacturing practice (GMP) divisions to improve compliance, increase clinical study robustness, and enhance data integrity.
The second article discussed pitfalls to avoid when developing GCP SOPs. This piece outlines the best approach to develop systems for selecting and monitoring vendors to ensure a high-quality study with data integrity — and minimal patient risk.
New therapy development is a long and complex process, and more sponsors are outsourcing clinical trial activities. Yet multiple reports from GCP regulatory inspections found major issues related to monitoring activities and data management, with CROs and sponsors accounting for nearly 43 percent of the total findings. To address these concerns, the International Council on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) released an amended version of the international guidelines for GCP: ICH GCP E6(R2).
The fundamental differences between the R1 and R2 versions of the guidelines relate to sponsor/investigator responsibilities and risk-based monitoring. In essence, the new regulations address concerns and questions about whether sponsors and CROs are taking enough care when assessing:
- how sponsors select and monitor vendors
- the execution process of a clinical trial
- data integrity, that is whether data submitted in the dossier is credible and accurate.
This article series has focused on how quality can collaborate with GCP and GMP groups to improve ICH E6(R2) compliance. However, in the case of vendor selection and oversight SOPs, I recommend separating GCP/GLP processes from GMP processes.
Although we think of it in a linear fashion, the process of vendor selection and qualification is not a linear process. The two can happen in parallel, with flexibility and required compliance and oversight. Steps can happen in parallel to facilitate and expedite the vendor selection process — and SOPs can be written in such a manner. You can move forward with a low amount of risk and build in flexibility that allows you to remain in compliance and avoid bottlenecks.
The flexibility comes from precise and clear SOPs. This allows each group to focus on specifics rather than vague, lumped-together generalities that can create inefficiencies, redundancies, training burdens, and “paralysis analysis” when attempting to follow long, complex SOPs. For GCP SOPs, one main issue that is different from GMP is GCP is responsible for the “transfer of obligations” (TOO) to the vendor.
Many companies combine the vendor selection process for GMP/GLP/GCP into one document, but they are all quite different processes. When grouped together, these processes become ripe for examination and inspection from regulatory bodies. As a result, we are seeing a heightened priority and urgency from the FDA to require clarity around the specifics of GCP procedures, such as TOO.
To improve the system to select and oversee vendors and processes, start with the following SOPs that separate GLP/GCP and GMP:
- Vendor selection SOP for vendors supporting GCP/GLP activities: This SOP includes clear expectations and requirements of the relationship with the vendor, which include the TOO that will be defined as part of the vendor contract.
- Vendor selection SOP for vendors supporting GMP activities: This SOP focuses on product and facility specifics.
- Vendor qualification SOP, which is driven by the sponsor’s quality department: This SOP outlines how to create and maintain the clinical audit plan (i.e., the processes for planning, conducting, and reporting clinical GCP vendor audits) to ensure reliability of data and the protection of subjects’ rights/safety. Quality owns the list of vendors and/or qualifies vendors if they are not on the list.
- Vendor oversight plan SOP, which describes the process for sponsor oversight of GCP/GLP vendors: This SOP describes the development, approval, and management of the vendor oversight plan used to manage clinical trial vendors. This ensures CROs and clinical trial vendors perform their trial responsibilities in a manner consistent with standards set by the sponsor, as well as GCP and the FDA and other relevant regulatory bodies.
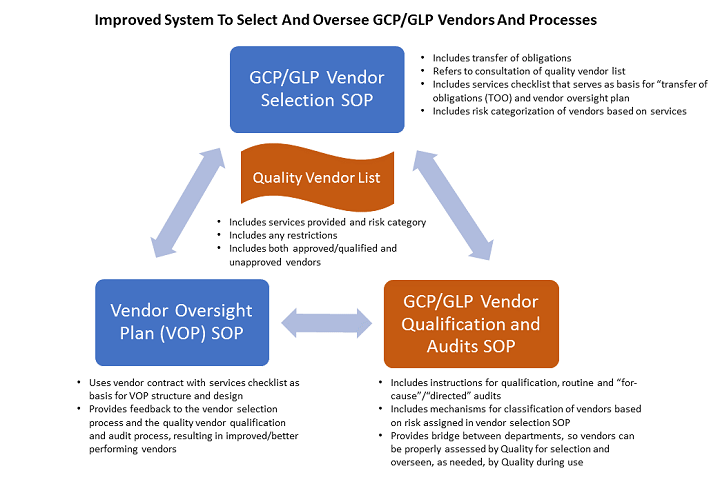
The vendor selection SOPs (#1 and #2 noted above) are similar in flow but contain different elements that justify separate processes. They begin with identifying the right people to bring to the table and completing a services checklist that provides the basis for constructing the RFP. The services checklist for GCP vendors is different than the checklist for GMP vendors since the services required of each are different.
Both selection processes refer to a vendor list, which quality owns and maintains, to determine if the vendor finalist(s) are quality approved/qualified vendors for the desired services. If not, the processes contain instructions for notifying quality of the intent to use a new vendor or a current vendor for new services and reference the associated vendor qualification SOPs. In this fashion, the vendor selection and qualification SOPs are linked together, which creates a more streamlined and collaborative approach.
The vendor qualification SOP (#3) is owned by quality assurance and provides instruction for categorization of each vendor type, based on risk with the associated criteria a vendor must meet to achieve a qualified status. If an audit is required, the timing for performing a requalification audit is also provided. Once the vendor is assessed by quality, the vendor list is updated, and quality notifies the stakeholders of the outcome. Again, quality is the link between the two processes and the GCP/GLP and GMP groups, improving process efficiency.
Based on the outcome of quality’s assessment, the vendor is selected, and the contract is negotiated. The vendor selection SOP instructs you to attach the completed services checklist to the contract as an appendix, since it outlines the TOO. Since the TOO is now part of the contract, you eliminate rounds of review and inspection associated with rechecking the services checklist and then going through the TOO again. It is all becomes part of the contract.
To then create the vendor oversight plan (#4), use the vendor contract(s) with the associated services checklist as a starting point. There are different approaches in designing a vendor oversight plan, but I recommend having a study oversight plan that outlines sponsor oversight of the operational activities outsourced to a CRO, and then creating separate appendices for other clinical development outsourced activities, which may not be assumed by the CRO (e.g., pharmacovigilance, translational medicine, and clinical pharmacology). With this structure, modifications can be made to each section without having to revise the entire plan, which makes the process more efficient and just as compliant.
Another important element of the vendor oversight plan is to identify which SOPs (sponsor or vendor) will be followed. Each section of the plan should include this in addition to the issues escalation plan.
Overall, the goal is to develop a seamless process with natural trigger points that involve quality personnel. When you change services with a provider, you go back to the contract and adjust these services, which should trigger both a consultation of the vendor list and possible notification to quality for assessment/qualification, and a revision to the vendor oversight plan to adjust oversight activities for the changed services.
The approach of separating GCP/GLP and GMP in vendor selection and oversight SOPs can lead to streamlined and efficient processes, which allow for better oversight, data integrity, and overall patient safety.
About the Author
 Judy Carmody, Ph.D., is the founder and principal consultant of Carmody Quality Solutions, LLC (CQS). She has 20+ years of expertise in applied technology, bench chemistry, analytical development, validation, quality management, and senior leadership. She has built quality management systems for both startup and Fortune 500 companies.
Judy Carmody, Ph.D., is the founder and principal consultant of Carmody Quality Solutions, LLC (CQS). She has 20+ years of expertise in applied technology, bench chemistry, analytical development, validation, quality management, and senior leadership. She has built quality management systems for both startup and Fortune 500 companies.
Carmody founded Avatar Pharmaceutical Services, an FDA-registered contract research organization that provided quality, submission-ready, customized analytical services in compliance with cGMP. She grew Avatar to 25+ employees and more than 75 clients before it was purchased by a Boston-based pharmaceutical company in 2010. She holds a Ph.D. in analytical chemistry from Clark University in Worcester, Massachusetts. Please connect with her on LinkedIn.
