Clinical Trial Feasibility: A Comprehensive Guide
Before sponsors launch a clinical trial, they often conduct a feasibility study to evaluate the proposed study and potential sites. A feasibility study weighs several factors: resources, time constraints, ethical considerations, and potential participant risks. By determining feasibility up front, sponsors avoid wasting money on unethical, impractical trials while protecting participants and increasing the chances of the study being completed successfully.
This comprehensive guide explains the components and nuances of feasibility studies and includes the following:
- Types And Levels Of Feasibility In Clinical Trials
- Key Components Of Clinical Trial Feasibility
- The Feasibility Assessment Process
- Challenges And Solutions In Clinical Trial Feasibility
- Advanced Techniques And Tools For Feasibility Assessment
- The Trend Of Leveraging RWD And RWE In Feasibility
- Frequently Asked Questions (FAQs)
Types And Levels Of Feasibility In Clinical Trials
Determining clinical trial feasibility is like moving through a funnel. Sponsors begin at the broadest program level, assess the particular study, and finally look for suitable sites. This incremental approach enables sponsors and researchers to make informed decisions that conserve resources and improve trial completion rates.
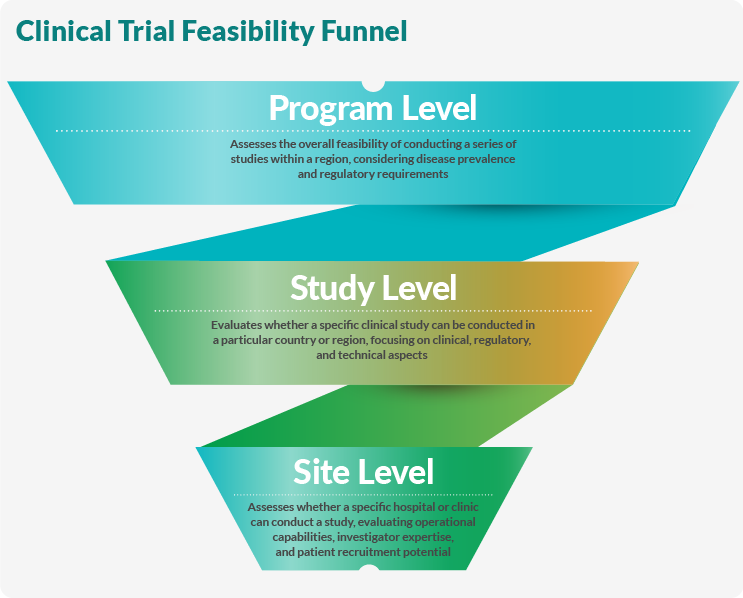
Program-Level Feasibility
Program-level feasibility looks at an entire research program or therapeutic area. This study measures disease or condition prevalence in a specific region relative to the population and gathers clinical and epidemiological information. Regulatory and ethical concerns are also examined at this stage, and existing treatment patterns and guidelines are considered. Examples of program-level feasibility studies include:
- Oncology: Measuring the prevalence of different types of cancers and their existing treatments in a certain country or region.
- Cardiovascular diseases: Studying heart disease epidemiology and the regulatory framework and healthcare infrastructure to conduct multiple cardiovascular studies.
- Rare diseases: Selecting a group of rare diseases and evaluating their prevalence, patient populations, and relevant ethical considerations.
- Vaccine development program: Estimating the feasibility of conducting multiple vaccine trials for different diseases in specific geographical areas.
Study-Level Feasibility
At the study level, researchers narrow their focus from broad populations and clusters of diseases or conditions to determine the feasibility of a specific clinical trial in a selected country or region. Researchers first look at epidemiological data for the study-specific population. Next, they evaluate the current standard of care, including availability and compatibility with the study's protocol.
Researchers also consider whether inclusion and exclusion criteria are workable with the given patient population. Regulatory requirements, approval timelines, technical aspects, and study-specific procedures are also carefully examined.
At this stage, researchers do what they can — while still maintaining scientific standards — to improve patient-centricity by incorporating participant needs and preferences into the trial design and protocol. Researchers should use feasibility studies to understand the patient's journey from symptom onset to treatment and consider the socioeconomic factors that affect their lives. Additionally, trial designs should minimize participant burden and include patient-relevant outcomes.
Study-level feasibility assessments could look like the following:
- Oncology study for a novel targeted therapy: Evaluate regulatory approval timelines, molecular testing facility availability, and whether study-specific procedures are acceptable in different regions.
- Hypertension trial focusing on mild-to-moderate patients: Determine patient recruitment potential, screening failure rates, and protocol-specific requirements like treatment washout periods.
- Rare disease trial: Measure the condition’s prevalence, identify possible study sites, and determine whether the study can meet recruitment goals.
- Vaccine study: For a particular geographic area, assess the disease epidemiology, regulatory requirements, site capabilities, recruitment potential, and the study’s technical aspects.
Site-Level Feasibility
Site-level feasibility is the narrowest part of the feasibility funnel. This type of study determines whether a particular hospital, clinic, or investigator can successfully conduct the study. First, the investigator's experience and readiness are evaluated. Next, researchers look at the site's patient population, recruitment potential, demographics, infrastructure, and resources such as staff, equipment, and technology.
Ideally, the site has a proven track record of successful clinical trials relevant to the specific trial or study. Below are examples of site-level feasibility studies:
- Oncology study: The site or investigator should have experience with specific cancer types and treatments, access to diverse patient populations, the capability to manage complex or adaptive protocols, and specialized equipment such as molecular testing capabilities or imaging equipment.
- Cardiovascular trial: For a cardiovascular trial, the site or investigator should be well-versed in cardiovascular studies and procedures, have access to patients with specific conditions, be able to follow up with patients over a long period, and have contingency plans for managing potential adverse events. Additionally, they should have cardiac monitoring equipment on hand.
- Rare disease trial: Most importantly, the site or investigator should have experience treating rare diseases and access to the appropriate patient population. They also need specialized diagnostic capabilities and the ability to manage complex protocols. Ideally, they also have established relationships with patient advocacy groups, which bolsters recruitment and patient-centricity.
- Vaccine study: A site or investigator wishing to lead a vaccine study should first have experience running vaccine trials and an established cold chain management strategy. These sites also need access to potential healthy participants or specific at-risk populations, long-term monitoring capacity, and suitable facilities for storing and administering the vaccines.
Key Components Of Clinical Trial Feasibility
Determining a clinical trial's feasibility is more than deciding whether or not to proceed with the study. Assessing the factors described below also helps sponsors optimize resource allocation and improve patient recruitment.
Patient Recruitment Feasibility
Recruiting participants is the first hurdle to completing a clinical trial. However, before sponsors begin their recruitment efforts, they should realistically assess the likelihood of enrolling the required number of participants within the desired timeframe. To determine this, sponsors and researchers first measure the prevalence of the target condition in the study region. Next, they look at historical recruitment data from similar studies, including screening failure and dropout rates. Finally, they consider various recruitment strategies and their effectiveness. For example, a study involving a common condition like hypertension would have an easier time finding subjects in a given geographical area than a rare disease study, which would have to cast a wider geographic net.
Operational Feasibility
Clinical trials require complex operations, so evaluating whether the study can be conducted with the existing operational setup, including technology and technical expertise, a site's capacity to manage complex protocols, and the feasibility of study timelines and procedures is important. For example, studies involving biologics such as cell or gene therapies must have cold chain logistics to manage sensitive materials.
Regulatory And Ethical Feasibility
Clinical trials must comply with local and regional regulatory bodies, which is particularly complex for trials conducted in multiple countries or regions. Sponsors should review local regulatory approval processes and timelines when planning a study. Additionally, the study must align with ethical guidelines, such as ensuring informed consent.
Protocol Design Feasibility
Protocol design is another issue that can make or break a study's feasibility. First, it must be compatible with the local standard of care. A complicated study should be closely evaluated to avoid unduly burdening participants. Finally, inclusion and exclusion criteria should be realistically assessed to ensure adequate enrollment.
The Feasibility Assessment Process
Assessing feasibility requires a detailed, logical strategy considering every aspect of conducting a clinical trial successfully. Establishing a comprehensive, objective plan of action enables sponsors to make well-informed decisions, conserve resources, and improve their trials' chances of success.
Pre-Assessment Planning
Preplanning sets the stage for a feasibility study and helps sponsors identify any obstacles to study completion. This stage entails several steps:
- Define objectives: Begin with clearly stated objectives for the study, e.g., “Determine if the study can recruit 500 hypertension patients in six months across 10 sites in the Northeastern United States.”
- Identify stakeholders: Create an interdisciplinary team to review the feasibility study, including specialists in clinical operations, medical affairs, regulatory requirements, logistics, and data analytics.
- Select sites: Compile a diverse list of potential sites based on performance history, location, and expertise. For example, for a rare disease study, select sites with experience with rare disease trials and connections to patient advocacy groups.
- Establish timelines: Realistic deadlines keep the assessment on schedule. For instance, researchers could allow two weeks for site responses, one week to compile data, and another week for analysis and reporting.
Data Collection And Evaluation
Determining feasibility relies on gathering comprehensive data on the following aspects:
- Epidemiology covers disease prevalence and patient demographics. For example, a vaccine trial feasibility study may examine seasonal variations in disease incidence.
- Site capabilities such as infrastructure, experience, staff expertise, and resources are considered.
- Regulations specific to the country or region affect feasibility. For instance, some countries require local clinical data for specific types of studies.
- Another key aspect is recruitment potential, including historical data and proposed recruitment strategies.
- Protocol feasibility asks if the protocol aligns with local practices. For example, a cardiovascular trial may require follow-up visits, and sponsors should determine if these visits are feasible for the local patient population.
Reporting And Decision-Making
After completing the stages detailed above, it’s time to make an informed decision regarding the proposed clinical trial. First, all data must be compiled and organized into a coherent, intelligible format. For example, companies can use spreadsheets or data visualization tools to compare site capabilities or projected recruitment rates.
Next, interpret the data to identify challenges and opportunities. For instance, the sites may have the necessary expertise, but the inclusion criteria may be too restrictive to meet the study’s enrollment goals. Once the data is collected and interpreted, researchers should compile a comprehensive report detailing findings and recommendations, such as a SWOT analysis for potential sites.
The feasibility stage of clinical trial development is a golden opportunity to improve the study before it starts. For example, if recruitment is an issue, expanding the inclusion criteria before the study begins can make recruitment more manageable. Sponsors could also consider adding sites in regions with higher disease prevalence. Once the report is compiled, sponsors can decide whether to proceed as planned, modify the protocol, or reassess the study.
Challenges And Solutions In Clinical Trial Feasibility
Assessing feasibility carries many challenges that should be mitigated by employing best practices.
Common Challenges
Common challenges feasibility assessments face include:
- Inaccurate data collection: Investigators may overestimate eligible patients and site capabilities.
- Time and resource constraints: Strict timelines and limited budgets can lead to feasibility studies that lack depth and quality.
- Lack of tailored approaches: Feasibility assessments often send sites generic questionnaires not specific to the trial or investigator.
- Incomplete information sharing: Commonly, sites are not given full protocol details and thus make incorrect assumptions.
- Balancing stakeholder interests: Multiple stakeholders with conflicting interests affect the scope and effectiveness of feasibility studies.
- Data reliability: Accurate, up-to-date, and unbiased data can be difficult to gather, especially when dealing with large volumes of information for complex projects.
- Regulatory complexities: Complying with disparate local, national, and international regulations complicates the feasibility assessment process.
- Bias towards experienced sites: Sponsors tend to focus on established sites, overlooking research-naive PIs who may have the right expertise for the study.
- Disconnect between stakeholders: If stakeholders are not communicating with each other, they may be unaware of the difficulties involved in the feasibility process.
- Pressure to confirm pre-existing decisions: Feasibility studies should be honest assessments rather than used to confirm pre-made decisions.
Solutions And Best Practices
Following these best practices improves the accuracy and effectiveness of feasibility studies, which ultimately improves trial execution:
- Improve data collection accuracy: Electronic medical record data offer precise patient population estimates, while statistical modeling and past performance data lead to better feasibility analyses.
- Optimize time and resource allocation: Use technology to centralize information exchange, streamline the process, and leverage existing tools and frameworks specifically designed for trial emulation with real-world data (RWD).
- Develop tailored approaches: Create customized feasibility questionnaires specific to each trial and give sites full protocol details to avoid incorrect assumptions.
- Enhance stakeholder collaboration: Engage sites during protocol development to align with local practices and collaborate with experts in RWD analysis and clinical trial design.
- Improve data reliability: Combine RWD analysis with traditional methods like investigator site visits. Look for potential biases in RWD and assess their impact on feasibility assessments.
- Address regulatory complexities: Develop a comprehensive understanding of country-specific regulations early in the planning phase. Collaborate with regulatory experts to navigate complex requirements.
- Expand site selection: Consider research-naive PIs to expand site options and employ a site-centric approach to improve trial outcomes.
- Align stakeholder interests: Clearly define the feasibility assessment team's roles, responsibilities, and timelines.
- Minimize bias in decision-making: Establish clear criteria for site selection based on objective data.
- Leverage technology: Explore opportunities in RWD/real-world evidence (RWE) and digital/decentralized trials (DCTs). Use centralized platforms for information exchange between sponsors, CROs, and sites.
Advanced Techniques And Tools For Feasibility Assessment
Advanced feasibility techniques and tools provide valuable insights and reduce potential risks, leading to more cost-effective, successful clinical trials.
Technology And Innovation In Feasibility
Innovative technology has greatly improved the feasibility assessment process by accelerating timelines and improving accuracy. These tools access diverse data sources, provide frequent updates as information changes, and are suitable for various disease indications.
- RWD platforms use patient data to identify eligible participants and potential trial sites.
- AI optimizes clinical trial design and creates crucial insights to improve enrollment and expedite studies.
- Electronic data capture systems automate processes that traditionally are performed manually, leading to faster, more accurate data collection.
- Clinical metadata repositories manage and standardize clinical trial data, improving efficiency.
- Mobile technologies, such as mobile devices and apps, provide remote data collection and monitoring to improve patient centricity and optimize data quality.
- Mapping software visualizes potential trial sites and patient populations to streamline the site selection process.
- Modeling and simulation tools predict trial outcomes and optimize trial design before the study begins.
- Site intelligence platforms construct dynamic scoring outputs using historical and predicted site performance.
Trial Simulation And Modelling
Clinical trial simulation (CTS) uses historical data to digitally model therapeutics, mechanisms, and disease-level information to predict trial outcomes and develop optimized protocols. Additionally, CTS can predict optimal dosing through each clinical trial phase, adjust doses for specific populations, and preemptively answer questions that would be impractical to address using traditional study methods.
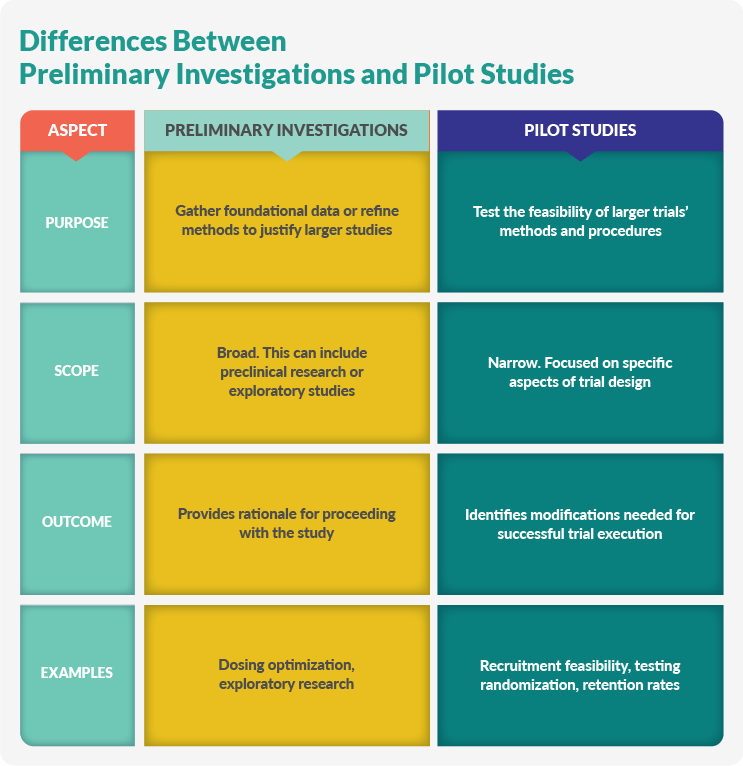
Preliminary Investigations And Pilot Studies
Preliminary investigations and pilot studies aim to reduce risks and improve research quality. Preliminary investigations gather background information, while pilot studies test specific trial methodologies.
Preliminary Investigations
Preliminary investigations gather foundational data or establish methods before researchers commit to a larger study:
- Optimization studies determine an intervention's appropriate dose, frequency, or duration.
- Feasibility assessments identify risks and refine methods before full-scale research.
- Exploratory research recognizes under-researched areas or populations to formulate key questions and support larger studies.
Pilot Studies
Pilot studies test the feasibility of large, comprehensive clinical trials and are suitable for qualitative and quantitative research. They identify potential issues so researchers can make necessary adjustments before the study launches. Pilot studies streamline research, enhance the study’s effectiveness, and enable researchers to refine research questions, methods, and data collection procedures. Examples of pilot studies include:
- Testing recruitment strategies, randomization processes, or participant retention rates.
- Determining whether a novel intervention can be implemented effectively within a controlled study environment.
- Evaluating assessment tools or data collection methods for reliability and practicality.
The Trend Of Leveraging RWD And RWE In Feasibility
Using RWD and RWE in clinical trial feasibility is a growing trend. RWD are gathered from electronic health records, claims databases, patient registries, and wearable devices. The raw data are analyzed to create RWE, which helps researchers better understand patient populations and enrollment projections and refine their recruitment strategies while providing more accurate predictions of the trial's outcome.
Conclusion
Assessing a trial's feasibility is a multistep process that examines the program, study, and individual sites for data-driven decision-making. These assessments can save sponsors and researchers time and resources by allowing them to identify and solve potential problems before the study begins. A comprehensive feasibility strategy is crucial to planning and executing today’s complex clinical trials.
Frequently Asked Questions (FAQs)
Below are frequently asked questions regarding clinical trial feasibility:
1. What is clinical trial feasibility?
Clinical trial feasibility evaluates whether a research study can be conducted practically and ethically at a particular site or region. It assesses factors like resources, patient population, timelines, and regulatory requirements to determine if a trial is likely to succeed.
2. Why is clinical trial feasibility important?
A feasibility assessment identifies potential challenges, ensures efficient resource use, protects participants from unnecessary risks, and increases the likelihood of study completion. It also helps sponsors and sites make informed decisions about proceeding with a trial.
3. What are the key components of a feasibility assessment?
Key components include evaluating patient population availability, site resources and capabilities, regulatory requirements, timeline feasibility, budget considerations, and alignment with local medical practices and guidelines.
4. Who is involved in the feasibility process?
The process typically involves sponsors, CROs, investigators, site staff, healthcare system leadership, IT personnel, and sometimes patient partners.
5. What types of feasibility assessments exist?
There are three main types: program-level (for an entire research program), study-level (for a specific clinical trial), and site-level (for conducting a study at a particular location).
6. How can sites improve their chances of being selected for clinical trials?
Sites can improve their chances by understanding sponsor requirements, evaluating their resources accurately, demonstrating strong patient recruitment potential, and showcasing their experience and capabilities in conducting clinical trials.
7. What are some common challenges in clinical trial feasibility?
Common challenges include unrealistic recruitment projections, inadequate site resources, regulatory delays, and misalignment between protocol requirements and local medical practices.
About The Author
Elizabeth Mann is a skilled writer with over a decade of experience in content creation, specializing in the life sciences industry. As a writer for Life Science Connect, she develops in-depth content that informs and engages professionals in the pharmaceutical, biotechnology, and medical device sectors. Her areas of focus include biologic drug production (including cell and gene therapies), clinical trial design and execution, and drug development and manufacturing outsourcing.
EXPERT INSIGHTS ON FEASIBILITY
-
As The Pendulum Swings, More Sponsors Initiate Site Identification And Feasibility
Consultant Megan Liles has seen outsourcing shifts over the years but says this one feels different. Here, Liles explains why she believes sponsors will ultimately initiate site identification and feasibility before bringing a CRO on board.
-
Why Sponsors Must Check For Site GCP Adherence During Feasibility Studies
GCP consultant Donatella Ballerini explores how adherence to GCP influences site feasibility, shaping the success and credibility of a clinical trial.
-
How To Determine Country Feasibility For Cell And Gene Therapy Clinical Trials
Learn how to choose the right location — globally — for your next cell and gene therapy clinical trial.
-
Site Feasibility Tips For Cell And Gene Therapy Clinical Trials
Site feasibility assessment can be challenging. Therefore, it’s essential to have a solid understanding of the regulatory obligations for investigators and sites when it comes to choosing each.
ON-DEMAND FEASIBILITY WEBINARS
-
Leveraging Internal And External Insights For Precision Enrollment Modeling
Discover a trial feasibility tool that integrates users' operational insights with high-quality clinical intelligence to drive accurate and informed enrollment projections and site capacity analyses.
-
Leveraging AI And Machine Learning To Deliver Next Generation Feasibility & Site Selection
In this presentation delivered at SCOPE 2022, hear how ICON leveraged Study Feasibility to its advantage.
-
Elevating The Site-Sponsor Experience: SaaS-Based Feasibility And Site Selection For Clinical Trials
In this webinar, learn about the transformation clinical trial feasibility and site selection across the industry.
-
There's (Got to Be) A Better Way – Managing The Feasibility Process
The site selection and feasibility process shouldn’t be so hard. It’s quite straightforward on paper. And yet, it is astonishingly difficult. During this webinar, we explored several answers to the question: Why does the study start-up process fail us so often? We’ll let you in on this secret answer – it might be how you are approaching the process itself.








