Introduction To Clinical Trial Management
Clinical trial management entails planning, executing, and overseeing drug or medical device studies to ensure safety, regulatory compliance, and data integrity. Protocol development, participant recruitment, regulatory adherence, data collection, monitoring, and reporting all fall under the clinical trial management umbrella. Effective trial management ensures that studies are ethically conducted, meet budget and timeline goals, and produce high-quality data. This comprehensive guide explains the fundamentals of clinical trial management, including:
- Roles And Responsibilities In Clinical Trial Management
- Key Components Of Clinical Trial Management
- Role Of Technology In Clinical Trial Management
- Challenges In Clinical Trial Management
- Frequently Asked Questions (FAQs)
Roles And Responsibilities In Clinical Trial Management
Clinical trial management requires a team of specialists who are responsible for different aspects of the trial and work together to ensure the trial is safe, compliant, timely, and cost-effective, including:
- Clinical trial managers are responsible for trial operations, meeting timelines and budgets, and stakeholder communication.
- Principal investigators oversee trial execution at the site, including following the protocol and supervising participant safety.
- Clinical research associates monitor trial site activities, verify data accuracy, and ensure regulatory compliance.
- Data managers administer trial data collection, storage, and analysis to ensure accuracy and integrity.
- Regulatory affairs specialists manage regulatory compliance and approvals.
- Project managers handle project planning, risk management, and resource allocation.
- Biostatisticians use statistical methods to analyze trial data and evaluate outcomes.
- Quality assurance teams ensure trial processes meet pre-determined quality standards.
Key Stakeholders In Trial Management
The following stakeholders each play a critical role in clinical trial management:
- Sponsors are pharmaceutical companies or academic institutions that finance and own the trial.
- Contract research organizations (CROs) are outsourced trial management and monitoring service providers.
- Ethics committees/institutional review boards (IRBs) safeguard participants’ rights and approve trial protocols.
- Regulatory agencies like the FDA and EMA approve trial designs and protocols and review study results for compliance and safety.
- Site staff, such as clinical coordinators and nurses, provide recruitment assistance and patient care.
- Patients/participants/ subjects take part in the study after giving explicit informed consent.
- Suppliers/vendors deliver medications, lab tests, or devices.
Key Components Of Clinical Trial Management
Clinical trial management encompasses the entire study lifecycle, from initial design to final analysis and submission to regulatory authorities. Effective management strategies balance scientific inquiry with pragmatism while prioritizing patient-centricity and data integrity.

Study Design and Planning
Managing a clinical trial begins at the study design stage and influences how and where the trial will be conducted.
Protocol Development
Clinical trial protocols establish the trial’s objectives, design, methodology, statistical considerations, and organizational structure while prioritizing participant safety, data integrity, and regulatory compliance. Protocols undergo rigorous review and include:
- Study objectives, both primary and secondary
- Trial design (e.g., single-arm, randomized control, master protocol, or medical device studies), intervention details, and duration
- Participant selection, i.e., inclusion/exclusion criteria
- Data management approaches for collecting, analyzing, and disseminating data
- Ethics and safety procedures, including the informed consent process and adverse event monitoring
- Regulatory compliance strategies based on local governing authorities such as the FDA or EMA.
Regulatory Approvals
Clinical trials must meet regulatory requirements for the country or region in which they are conducted. The FDA, EMA, and other agencies review the trial protocol and supporting documents to ensure compliance before the trial begins. Although regulatory details differ from agency to agency, typical requirements include informed consent, adverse event reporting, and data management strategies that conform to local patient privacy laws. Sample regulations include:
- FDA Clinical Trials Guidance Documents
- EMA Clinical Trials Regulation
- WHO Guidance For Best Practices For Clinical Trials
Ethics Review
Ethics committees like IRBs evaluate and monitor the trial throughout its lifecycle to ensure participant rights and welfare, including:
- Measuring the study’s risks vs. benefits
- Assessing informed consent processes
- Monitoring good clinical practice (GCP) compliance
- Reviewing protocol amendments, adverse event reports, and participant safety data.
Site Selection
Site selection is critical in clinical trial management and affects recruitment, data collection, and study timelines. Sponsors conduct feasibility assessments to evaluate site readiness through standardized questionnaires or predictive models and weigh the following factors:
- Geographical location to reach the target population
- Site capabilities, such as infrastructure, staff expertise, and clinical trial track record
- Participant recruitment potential using epidemiological data.
Budget And Financial Oversight
Budget and financial oversight ensures that the study is financially viable and that resources are efficiently allocated throughout the trial’s lifecycle. Given the high cost of clinical trials, developing, distributing, managing, and auditing resources is vital to trial success.
Budget Development And Allocation
First, the study’s budget is carefully developed and allocated. This entails estimating costs for personnel, study-related procedures, investigational products (IPs), site fees, and regulatory compliance. High-impact budget items like patient recruitment, staff salaries, and data management are prioritized, and cost-benefit analyses ensure that each budgeted item adds value.
Financial Management Tools
Specialized tools improve budget oversight. For instance, clinical trial management systems (CTMS) streamline financial processes, automate data recording, and give real-time access to financial information, engendering rapid risk mitigation and better control over the budget.
Regular Budget Reviews
Ongoing financial oversight through regular budget reviews identifies financial inefficiencies, closely tracks expenses to detect unforeseen costs, and adjusts allocations based on the trial’s progress and changing circumstances.
Financial Audit Process
A robust financial audit process includes understanding pre-award budgets, setting up calendars and invoice terms in the CTMS, and scheduling regular audit meetings to improve cost-effectiveness.
Following Accounting Principles
Financial oversight should adhere to generally accepted accounting principles (GAAP) for transparency and accountability. GAAP compliance helps maintain accurate financial records and reporting, facilitates improved financial decision-making, and helps secure future funding opportunities.
Invoicing And Revenue Recognition
Timely and accurate invoices ensure steady cash flow, improve relationships with sites and vendors, and maintain the trial’s financial health.
Regulatory Compliance
Regulatory compliance commonly entails following GCP guidelines, conducting external and internal audits and inspections, and adequately managing protocol amendments.
GCP standards are internationally recognized and describe how clinical trials should be designed, conducted, recorded, and reported. Participant safety and well-being are prioritized, and data integrity is paramount. GCP details investigators’ sponsors’, monitors,’ and ethics committees’ responsibilities. The FDA requires investigational new drug applications (INDs) or investigational device exemptions (IDE) to comply with GCP guidelines.
Audits and inspections ensure regulatory compliance and maintain clinical trial quality. Internal and external audits assess the study’s adherence to protocol, GCP guidelines, and regulatory requirements. Audits also evaluate whether participants’ safety and rights are protected and check trial data for accuracy, reliability, and verifiability. Inspections, by contrast, are conducted by regulatory authorities to evaluate compliance. Sponsors and investigators must proactively prepare for audits and inspections to avoid issues like delays or regulatory actions such as warning letters or clinical holds.
Protocol amendments (i.e., change order) are common in clinical research, particularly for adaptive or master trial designs. New information or unforeseen circumstances prompt protocol amendments, but any changes must align with the trial’s scientific goals and participant safety standards. These amendments must be submitted to ethics committees and regulatory bodies like the FDA for review before implementation.
Participant Recruitment And Management
Participants are the lifeblood of clinical research, but poor recruitment and retention rates continue to plague the industry, resulting in costly delays or trial failures. Clinical trial management includes creating targeted recruitment strategies that leverage digital tools and engage healthcare providers to identify suitable subjects.
Managing participants and keeping them engaged in the trial is equally critical. Clear communication, flexible scheduling, and addressing participants’ needs can reduce dropout rates. Strategically implementing a patient-centric approach ensures compliance, improves retention, and fosters trust.
Monitoring And Oversight
 Monitoring sites and managing the trial master file (TMF) are necessary for regulatory compliance.
Monitoring sites and managing the trial master file (TMF) are necessary for regulatory compliance.
Site monitoring ensures that trials comply with the approved protocol, GCP guidelines, and regulatory requirements. It entails regular communication with site staff, process review, data verification, and checking documentation procedures. Monitors (i.e., CRAs) visit sites in-person or remotely to evaluate participant enrollment, informed consent procedures, IP management, and protocol adherence. Effective monitoring protects participants, maintains data integrity, and flags potential protocol or regulatory compliance issues.
The TMF stores the essential documents demonstrating GCP and regulatory compliance throughout the trial. Documents include those prepared before, after, and during the trial, including protocols, informed consent forms, and audit certificates. TMFs should be transparent and allow trial conduct to be reconstructed if needed.
Risk Management
Potential risks must be identified, assessed, mitigated, and monitored to protect participant safety, data integrity, and regulatory compliance. A risk-based monitoring (RBM) approach systematically evaluates critical-to-quality factors like safety endpoints and data processes to prioritize resources and reduce risks. Proactive risk identification should occur before the study begins and include tailored mitigation strategies. Effective risk management reduces operational inefficiencies, prevents protocol deviations, and enhances trial quality by addressing vulnerabilities early in the study.
Data Management
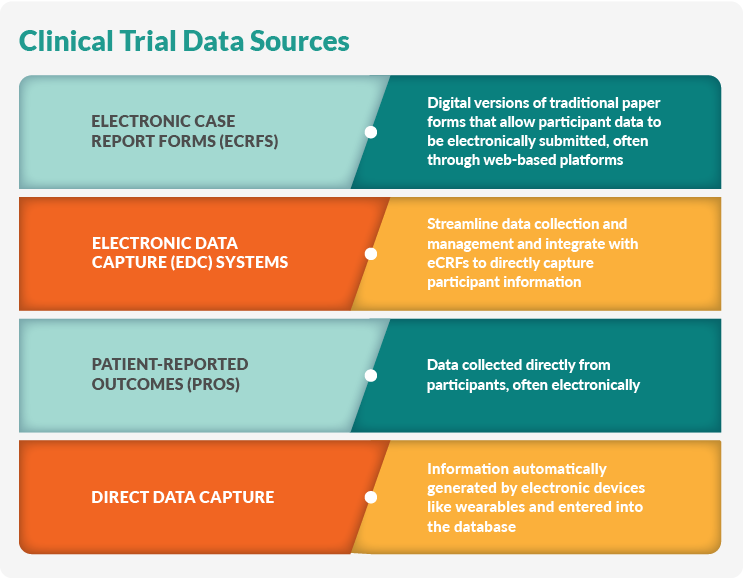
Data management strategies protect the study’s integrity, accuracy, and reliability. First, data are gathered from various sources and methodologies, including:
- Electronic case report forms (eCRFs) are digital versions of traditional paper forms that allow participant data to be electronically submitted, often through web-based platforms.
- Electronic data capture (EDC) systems streamline data collection and management and integrate with eCRFs to directly capture participant information.
- Patient-reported outcomes (PROs) are data collected directly from participants, often electronically.
- Direct data capture is information automatically generated by electronic devices like wearables and entered into the database.
Next, data quality control management systems ensure accuracy, integrity, and consistency throughout the trial. For example, regular data trend analysis identifies outliers and deviations. Data should be periodically reviewed during the study, and EDC systems enable real-time data checks. Meanwhile, RBM approaches focus resources on the most critical data points.
In particular, adverse events must be identified, assessed, and documented promptly. A robust data management system allows researchers to quickly assess adverse events and report them to relevant stakeholders such as sponsors, ethics committees, and regulatory authorities.
Clinical Trial Supply Management
A robust clinical trial supply management strategy ensures that IP and other materials are delivered on time to trial sites while meeting regulatory requirements.
IP, such as drugs, biologics, or medical devices, are carefully managed throughout their lifecycle, including manufacturing, packaging, labeling, storage, and distribution. IP management must comply with GMP standards, which cover handling, storage conditions, and accountability, which are documented via an audit trail. IP management also includes training site staff on administration protocols and managing randomization or unblinding procedures to maintain trial integrity.
Clinical supply logistics delivers trial materials to sites while navigating challenges like cross-border regulations and temperature-sensitive shipments. Demand forecasting, inventory management, real-time shipment tracking, and compliance with good distribution practices are all crucial to preventing delays or disruptions. Advanced systems and global networks help deliver supplies accurately and on time, even for multisite trials.
Statistical Analysis and Reporting
Statistical analysis and reporting systematically evaluate and present trial data so researchers can draw meaningful conclusions. Statistical reports must be clear and accurate, using tables, figures, and listings to summarize the IP’s administration, efficacy, and safety.
Primary and secondary endpoints should be clearly defined to prevent type 1 errors (false positivity), and appropriate statistical methods should be documented in detail for validation and regulatory compliance.
Stakeholder Communication
Clinical research involves many stakeholders, including sponsors, researchers, study coordinators, participants, community members, and regulatory bodies; miscommunication can cause delays or waste resources. A comprehensive communication plan should build trust and respond to misinformation while transmitting the study’s objectives, protocols, and timelines. Engagement roadmaps and multi-stakeholder recommendations should involve all stakeholders from the earliest stages of trial design. Additionally, communication strategies must be flexible and adapt to the study’s evolving needs and circumstances.
Role Of Technology In Clinical Trial Management
Manual processes and paper-based record-keeping are being replaced by advanced digital technologies that are more efficient, accurate, and timely.
Clinical Trial Management Systems
CTMS platforms streamline and support clinical trial management by centralizing and organizing trial operations, such as:
- Protocol and study documentation management
- Site and investigator management
- Patient recruitment and enrollment tracking
- Budget and financial management
- Regulatory document control and eTMF management
- Visit scheduling and monitoring
- Data collection and quality control
- Reporting and analytics capabilities
CTMS facilitates stakeholder collaboration, automates processes, improves resource allocation, and enables real-time data access through these functions. They also help maintain regulatory and GCP compliance and audit preparedness while integrating with EDC systems and safety reporting tools. Given the complexity of today’s clinical trials, CTMS have become essential tools for sponsors and CROs.
Data Collection Technologies
Clinical trial data collection has progressed from paper-based forms to digital capture and management systems and continuous tracking from wearables and remote devices. A clinical trial management strategy includes planning for collecting and securing data while protecting participant privacy and complying with regulations.
Electronic Data Capture And eCRF Systems
EDCs comprise a comprehensive clinical data management ecosystem that streamlines data review, cleaning, and reporting. They facilitate real-time data collection and submission, centralized access for sponsors and CROs, and improved data quality with automated checks.
Meanwhile, eCRFs are digital forms that replace traditional paper forms. They are designed to minimize data entry errors, support cross-trial standardization, and enable rapid data analysis and monitoring.
Other e-Related Tools
Today’s trials generate more data than in decades past, which can burden sites. However, these clinical trial technologies improve data quality and engage participants while reducing site burden:
- eConsent involves the use of tablet-like devices (rather than multiple paper forms) to deliver patient-consent-related materials for a trial.
- ePRO (electronic patient-reported outcomes) enables participants to report symptoms or side effects or collect information through wearables.
- eCOA (electronic clinical outcome assessment) digitally captures outcome measures.
- eSource tools offer real-time remote data sharing, which improves stakeholder collaboration.
HIPAA And General Data Protection Regulation Compliance And Data Integrity
The FDA requires that clinical trials follow HIPAA privacy and data integrity rules. Researchers must create robust safeguards for handling protected health information and obtain written authorization from participants. Additionally, site staff should receive regular HIPAA training, and researchers should perform risk assessments and audits to identify vulnerabilities in their data management system.
Similarly, the EMA requires clinical trials to comply with General Data Protection Regulation (GDPR) mandates, which dictate how personal data can be used in clinical trial publications through rigorous anonymization techniques. Individuals, however, are granted access to rectify, delete, or restrict the processing of their data. GDPR also details data security measures.
Emerging Technologies
Emerging technologies enhance clinical trial efficiency, data quality, and participant engagement.
AI And Machine Learning For Predictive Analytics And Patient Recruitment
AI and machine learning (ML) are transforming clinical trials in numerous ways. First, predictive models analyze vast datasets to identify suitable candidates, boosting recruitment potential. They also enhance trial design by simulating outcomes and improving protocols. These tools enable adaptive trial designs that modify protocols in real-time based on interim results. Finally, AI and ML accelerate data analysis, allowing researchers to identify trends and correlations quickly.
Integration Of Wearable Devices For Real-Time Data Collection
Wearables like smartwatches continuously track vital metrics and provide real-time monitoring that detects fluctuations or trends that otherwise might go unnoticed. These devices reduce the required number of site visits, improving patient-centricity while collecting rich, longitudinal data sets.
Blockchain For Enhancing Data Security And Transparency
Blockchain technology provides a tamper-proof record of all transactions, improving transparency in clinical trial processes, facilitating secure data sharing, and protecting data integrity and traceability.
Cloud-Based And Mobile Solutions
Cloud-based and mobile solutions are improving data quality and patient-centricity. First, cloud computing offers centralized data management, shortening timelines and reducing costs while providing stakeholders real-time data access to make decisions quickly. Data security and compliance are also improved via robust auditing.
Mobile technologies like ePRO and eCOA provide remote, real-time participant monitoring and data collection, which reduces the number of required site visits. These technologies improve participant engagement and adherence to treatment protocols. Mobile tools also streamline data flow between sites and participants.
Integrating cloud-based and mobile solutions can improve operational efficiency through standardized workflows and real-time visibility, enhance data quality, accelerate analysis and decision-making, and simplify study planning and oversight.
Challenges In Clinical Trial Management
A common challenge in the industry is coordinating multisite clinical trials. Spreading trials over multiple sites, regions, or countries can offer recruitment and cost advantages, but multisite trials carry many challenges that must be addressed through careful planning, clear communication channels, and standardized training. Centralized management systems help sponsors run multisite trials efficiently across regions or countries.
Complexities Of Multisite Trials
First, multinational trials juggle separate regulatory agencies and ethics committees or IRBs to comply with local and international rules and regulations. Negotiating these requirements is particularly difficult when trials span different countries with diverse regulatory environments.
Operational challenges are another aspect of running multisite trials. Workflows and procedures must be standardized across sites to maintain consistency. However, managing site selection, staff training, and supply chains across regions or countries is complex. Biopharmaceutical products add another layer of complexity if they are shipped internationally. Finally, negotiating contracts with multiple sites can delay studies.
Data management and quality control must also be consistent across sites to protect data integrity. Managing real-time data sharing and analysis from multiple sources requires sophisticated technological solutions.
Communication and collaboration are vital to trial success, even when sites are in multiple locations and time zones. Multi-institutional studies require close cooperation between sites and academic partners. Clear and consistent communication strategies ensure that sites are aligned in their understanding and execution of the trial protocol.
Operating multisite trials increases recruitment opportunities but also introduces complexities. First, setting and meeting recruitment goals across diverse populations and areas requires local knowledge and targeted strategies. Managing retention consistently across sites is necessary for data validation. Resource management and mitigation strategies for staff turnover help keep trials running smoothly despite unexpected disruptions.
Conclusion
Clinical trial management engages every stage in a study’s design, execution, analysis, and regulatory submission. Industry trends are moving towards advanced technologies and improved patient-centricity as solutions to the ongoing challenges of low recruitment and lengthy timelines. Strategic management is essential to conducting meaningful, actionable research that brings new therapeutics and medical devices to market.
Frequently Asked Questions (FAQs)
Below are frequently asked questions regarding clinical trial management:
1. What is clinical trial management?
Clinical trial management refers to overseeing and coordinating all aspects of a clinical research study, from initial planning to final analysis and reporting. It ensures trials are conducted efficiently, ethically, and in compliance with regulations while prioritizing patient safety and data integrity.
2. Why is effective clinical trial management critical?
- It ensures compliance with regulatory requirements
- It protects participant safety
- It maintains data quality and integrity
- It optimizes resource allocation and efficiency
- It facilitates the development of new therapies and medical knowledge
3. What are the key components of clinical trial management?
- Planning and design
- Financial oversight
- Regulatory compliance
- Participant recruitment and management
- Monitoring and oversight
- Risk management
- Data management
- Clinical trial supply management
- Statistical analysis and reporting
4. Who are the main stakeholders in clinical trial management?
- Sponsors
- Investigators
- Participants
- Regulatory agencies
- Clinical trial management teams
5. What are some emerging trends in clinical trial management?
- Integration of advanced technologies like AI and wearable devices
- Use of real-world data and adaptive trial designs
- Enhanced focus on patient engagement and experience
6. How is technology changing clinical trial management?
- Enabling remote monitoring and data collection
- Improving data quality and analysis through AI and machine learning
- Enhancing patient recruitment and retention through digital platforms
- Streamlining processes with integrated eClinical ecosystems
7. What are the challenges in managing multisite clinical trials?
- Coordinating activities across different locations and time zones
- Ensuring consistent protocol implementation across sites
- Managing regulatory compliance in different jurisdictions
- Maintaining data consistency and quality across sites
8. How can clinical trials become more patient-centric?
- Involving patients in trial design and planning
- Using clear, simple language in communications
- Offering flexible participation options (e.g., remote visits)
- Considering patient-reported outcomes in trial endpoints
- Designing protocols that minimize disruption to patients’ daily lives
9. What is the role of data management in clinical trials?
- Collecting and storing data accurately and securely
- Ensuring data quality through validation and cleaning processes
- Managing data analysis and reporting
- Maintaining compliance with data protection regulations
- Supporting decision-making throughout the trial
10. How are regulatory requirements addressed in clinical trial management?
- Staying informed about current and evolving regulations
- Implementing robust quality assurance processes
- Ensuring proper documentation and record-keeping
- Conducting regular audits and inspections
- Training staff in regulatory compliance
- Engaging with regulatory bodies throughout the trial process
About The Author
Elizabeth Mann is a skilled writer with over a decade of experience in content creation, specializing in the life sciences industry. As a writer for Life Science Connect, she develops in-depth content that informs and engages professionals in the pharmaceutical, biotechnology, and medical device sectors. Her areas of focus include biologic drug production (including cell and gene therapies), clinical trial design and execution, and drug development and manufacturing outsourcing.
EXPERT INSIGHTS ON TRIAL MANAGEMENT
-
Should We Still Be Using Trackers For Clinical Trial Management?
While excitement abounds for AI in clinical research, many professionals are still using manual trackers and logs to record data related to monitoring visits, protocol deviations, and more. TMF consultant Donatella Ballerini discusses the relevancy of tracks and logs today.
ON-DEMAND TRIAL MANAGEMENT WEBINARS
-
Clinical Trial Management System
Watch to learn the reasons why research sites, site networks, health systems, academic medical centers, and cancer centers use a CTMS to centralize information related to protocols, subjects, staff, financials and billing, and much more.
-
ICH E6(R3): Practical Steps For Implementation
Watch this presentation to equip stakeholders with actionable strategies and a comprehensive understanding of the evolving clinical trial management landscape in the context of ICH guidelines.
-
New Paradigm For Clinical Management Systems
The usual approach of adding technology and headcount to manage today’s clinical trials is not sustainable or scalable. In this webinar, Nick Dyer (CEO, Catalyst Clinical Research) discusses the challenges his team has experienced with complex early phase oncology studies, and why they chose Medidata’s CTMS to help Catalyst attain operational success.
-
CTMS & Study Data: 2 Keys To Creating Optimal Process Transparency
Clinical trial management systems can either make or break operational workflows in medical studies. Listen to this discussion with Hope Weisser, Sr. Product Manager at TransPerfect, about the new features of Trial Interactive’s CTMS.
-
Empowering Teams For Successful Clinical Trial Management
Speakers provide insight into how clinical teams can better manage their drug development studies and associated trial master files to bolster the advancement and management of clinical research.





