RECENT WEBINARS
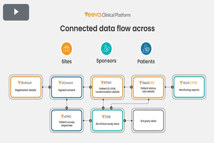
CLINICAL TRIAL TECHNOLOGY
E-TECHNOLOGIES
2:21
![GettyImages-1411002362 trial tech GettyImages-1411002362 trial tech]()

DATA MANAGEMENT
TRIAL MANAGEMENT
REGULATIONS & COMPLIANCE
CLINICAL LEADER SOLUTIONS EXPO
16:28
![Mural Recruitment October 2025 Mural Recruitment October 2025]()

16:47
![Clinical Leader Solutions Expo Clinical Leader Solutions Expo]()

PATIENT CENTRICITY
PATIENT RECRUITMENT & RETENTION
SITES
CLINICAL LEADER LIVE
OUTSOURCING
DECENTRALIZED TRIALS
1:03:01
![IQVIA ACT IQVIA ACT]()

TRIAL MONITORING
33:11
![phlex heatmaps thumb phlex heatmaps thumb]()

MOST RECENT STREAM CLINICAL LEADER LIVE VIDEO
53:53
![AI In Clinical Trials: What's New & What's Hype? AI In Clinical Trials: What's New & What's Hype?]()

AI In Clinical Trials What's New And What's Hype
Our third Clinical Leader Live on AI will go beyond the buzzwords to focus on what’s actually working in clinical operations.