
OUTSOURCING

Early Phase Clinical Development Solutions
Our experts in the design and conduct of clinical pharmacology and early efficacy studies ensure that data generated meets your objectives.

A Different Kind Of CRO
Altasciences is an award-winning, forward-thinking, mid-size early phase contract research organization, in Canada and the U.S., offering biopharmaceutical companies of all sizes a proven, flexible approach to early drug development.

How To Take Advantage Of Early Access Programs For The EU
Learn how to leverage Early Access Programs (EAPs) in the EU to expedite market entry for innovative life science products. Gain insights into running successful EAPs, including crucial steps for France.

Improving Sponsor Oversight Of The Trial Master File
In the face of increasing documentation requirements and trial complexity, discover how global biopharmaceutical company Chiesi and its CRO partners collaborated to ensure ongoing oversight compliance.

Navigating The Unique Attributes Of Psychedelic Drug Development
Join our panel of experts for an in-depth review of the FDA's guidance on psychedelic drug development, exploring necessary methodological adaptations for safety, pharmacology, and efficacy.

Imagine… Easier, Proactive Drug Development
Imagine partnering with a single CRO/CDMO for your early phase drug development. With Altasciences, you don’t have to imagine—learn how they’re able to deliver for your project.

Successful Implementation Of A Drug-Device Combination Submission
Projects including both drug and device components require strategic planning for smooth initiation. Regulatory considerations are the key to success.

Why Functional Outsourcing Is Important To Clinical Development
Craig McIlloney, Senior Vice President of Catalyst Flex, discusses why functional outsourcing is important in today's clinical development environment, how Catalyst Flex supports this, and is scalable to flex to your changing needs.
An Innovative Approach To Functional Outsourcing
The shifting dynamics of clinical research from the current funding dynamic to the increased complexity of drug development, requires a different approach to functional outsourcing to be successful.

Successful Manufacturing Of Clinical Trial Supply
Formulation and pharmacy experts share their secrets for completing clinical trials and resolving issues that could impact your drug development program’s timeline.

Outsourcing Clinical Trial Disclosure Management
Deciding to outsource clinical trial disclosure management is a sponsor’s first step. Deciding what processes to outsource is the next step. Two study sponsors discuss with TrialScope what they outsource and why. Watch the short video.

Altasciences Clinical Services At A Glance
We can support your entire drug development program end to end, or you can partner with us for just one element. We offer you complete flexibility.

We Are Altasciences: Proactive Drug Development
When you partner with Altasciences, you get a single source outsourcing partner who knows your program from end to end and who can anticipate your needs, for a seamless and simplified experience.

Getting Buy-In For Disclosure As A Service
Clinical trial disclosure teams often have a tough time convincing upper management on the benefits of Disclosure as a Service. Hear how two sponsors “sold” the idea of outsourcing this function. Watch the short TrialScope interview.
Altasciences Has You Covered From Coast To Coast
With your success in mind, we designed an integrated early phase drug development offering with eight locations, to get you from lead candidate selection to clinical proof of concept, and beyond.

Proactive Drug Development: Explained
Watch this short video to learn about Proactive Drug Development with Altasciences.

Big Impact With A Personal Touch
Transforming lives. Improving human health. Bringing new therapies to life. Altasciences' CRO and CDMO services deliver.

The Heart Of The Matter: Early Vs. Late QT Prolongation Testing
Dr. Beatrice Setnik, Altasciences' Chief Scientific Officer, walks through the differences between early and late QT prolongation testing.

Flipping The Drug Development Industry On Its Head
The video dives into the obstacles of the traditional outsourcing relationship between CROs and sponsors, and how Altasciences has broken from tradition to offer an alternative model.
Streamlining Patient Access To Cell And Gene Therapies
Learn about the obstacles patients encounter when seeking CGTs and explore how technology and patient services can empower manufacturers and clinical teams to help patients overcome barriers.
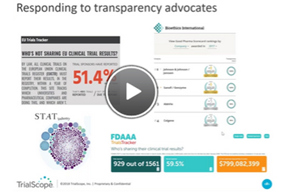
How To Boost Disclosure Efficiencies With Outsourcing
In this webinar, you’ll hear about exciting new advisory and managed disclosure services from TrialScope, including: compliance, policy and process assessments, as well as plain-language summaries, protocol registration, results posting, and redaction services.
...
How Sponsors Turn Disclosure As A Service Into A Strategic Advantage
In this panel discussion, Teva and Lung Biotechnology disclosure experts discuss why they opted for disclosure as a service. They will detail how it helps them safeguard regulatory compliance and mitigate risk.

The Path Forward, Altasciences' Approach To Project Management
Learn about a unique, integrated program management strategy that can lower your costs and reduce program timelines from lead candidate selection to clinical proof-of-concept by up to 40%.

Trends In Sponsor-Of-Choice Initiatives: Consolidating Site Payments
Listen in as leaders from Bristol Myers Squibb, CenExel Centers of Excellence, and IQVIA Technologies discuss the importance of providing a consistent clinical trial payment experience to sites.

See How You Can Start Your Early Phase Program 6-9 Weeks Earlier In Canada
Vice President of Business Development Lisa Sanford explains whether you need an IND before starting your first-in-human trial in Canada.
