A Guide to Patient Recruitment in Clinical Trials
“If you build it, they will come” might be a winning strategy for baseball fields in Iowa, but unfortunately, the same cannot be said for clinical trials. Recruitment, enrollment, and retention pose serious challenges industry-wide. Sponsors need proven strategies to overcome the odds and successfully recruit and enroll sufficient participants for their clinical trials.
To enroll participants in clinical trials, sponsors must identify, attract, and select suitable subjects. This critical process is challenging, often facing issues such as a lack of awareness, stringent eligibility criteria, and popular misconceptions about clinical research ethics.
Given these factors, 55% of trials worldwide fail due to insufficient enrollment, and only an average of 40% of Phase 3 trials experience enrollment success. In the U.S. alone, over 80% of trials fail to meet enrollment requirements and a 30% average attrition rate. Nevertheless, the importance of patient recruitment can not be overstated, as clinical trials are necessary for successful drug development. A successful trial recruitment strategy recognizes these challenges and relies on best practices and top-notch technology to meet them.
This comprehensive guide assists sponsors in developing a realistic strategy that encompasses the entire recruitment and enrollment process, with an emphasis on prioritizing patient-centricity and regulatory compliance.
Table of Contents
- Understanding Patient Recruitment
- Preparing For Patient Recruitment
- Strategies For Patient Engagement
- Enhancing Patient Experience During Enrollment
- Monitoring And Evaluation Of Recruitment Efforts
- Retention Strategies For Participants
- Regulatory And Ethical Considerations
- Leveraging Technology And Tools In Patient Recruitment
- Future Trends And Innovations In Patient Recruitment
- Selecting The Best Patient Recruitment Approach
- Frequently Asked Questions (FAQs)
- References
Understanding Patient Recruitment
Sponsors forecast and manage a probable randomization timeline as well as necessary parameters for successful recruitment. Factors such as available patient population, eligibility criteria, recruitment methods, and operational feasibility are weighed to determine if enough participants can be enrolled in the trial in the desired timeframe.
However, what determines "feasibility" is in the eye of the beholder. The exact definition can vary widely between sponsors, researchers, C-suite executives, site staff, and recruitment specialists. Sponsors must define feasibility for their study up front to determine whether the trial can move forward. For example, a rare disease study will have different feasibility criteria than a study tackling a widely prevalent disease.
The following criteria should be considered when determining a study’s feasibility:
- target demographics
- eligibility criteria
- disease incidence/ prevalence
- regulatory requirements
- patient access and awareness.
Study design also affects feasibility. Protocol design and operational factors that could affect recruitment are also weighed. For instance, a protocol that requires a significant time and travel burden for participants should not have the exact expectations for enrollment as a trial that patients can essentially complete outside of site visits.
Key Challenges In Patient Recruitment
Why is it difficult to recruit enough patients for clinical trials? There is no single answer to this question, but many common factors are at play, including:
Understanding Patient Diversity Requirements
Despite regulatory changes over the last few decades, women and minorities are still consistently underrepresented in clinical research. For example, in 2021, people of color comprised 39% of the U.S. population but only represented 2-16% of patients in clinical research. Lack of diversity diminishes the science of clinical research, resulting in less information about how a therapeutic affects a broad population. For these reasons, the FDA is changing its diversity requirements to ensure that clinical trial participants accurately reflect the targeted patient population’s demographics.
Diversity can be difficult to achieve, however, for several reasons, including:
- overly restrictive eligibility criteria
- lack of awareness/ access to clinical trial information in underserved communities
- cultural distrust of the medical system, particularly clinical research.
To meet these challenges, sponsors should set intentional recruitment goals to meet evolving diversity requirements. They can use patient registry data sets to directly recruit patients from underrepresented groups. Another solution is to expand recruitment beyond academic medical centers to community settings, such as local clinics. And finally, targeted outreach and engagement strategies that build trust and encourage participation help solve the diversity puzzle.
Impact Of Disease Prevalence And Incidence On Recruitment
Simply put, the rarer the disease, the harder it is to recruit adequate patients for clinical research. For example, common conditions like diabetes affect millions of patients globally and thus offer sponsors an enormous pool of potential participants. However, recruiting for rare diseases is not so easy. Enrolling dozens, much less hundreds, of patients in these lifesaving trials is significantly tricky and a common cause of trial failure. Fortunately, recent developments such as telehealth platforms and wearable devices have expanded patient recruitment for many rare disease studies.
Improving Patient Awareness And Access To Clinical Trials
You know your patient population, but do they know you? Even in our world of omnipresent information, the answer is that surprisingly few patients understand how participating in clinical research could benefit them. Outreach initiatives like educational websites and targeted campaigns help spread awareness. Adequate patient compensation also aids recruitment efforts. Additionally, emerging solutions such as ML and AI show promise in identifying and targeting eligible patients.
Sponsors should also consider how study design affects patients. Even if patients are willing to participate, site location and time burdens can prevent them from enrolling. Sponsors should look for ways to make trials more convenient for participants, both in terms of location access and protocol design.
Navigating Regulatory And Ethical Considerations
Informed consent is the first factor sponsors consider when navigating the regulatory and ethical landscape of patient recruitment. Fully informed consent in clinical trials is more than getting patients to sign a form. It includes in-depth education on the processes, requirements, and risks inherent in the study. Consent must also be re-obtained when protocol changes prove necessary. Educating patients can be as simple as in-office visits with the PI to review procedures and risks or as high-tech as online learning tools such as videos and other materials in patients’ native languages.
Institutional Review Board (IRB) approval is required before a study begins to ensure that all human trials are conducted ethically and humanely. IRBs are diverse and contain non-scientists and community members representing patient interests and wellbeing. These boards provide a number of oversights that affect patient recruitment, including:
- Review and approval: the IRB reviews and approves the clinical trial protocol, informed consent documentation, and related materials prior to the study launch. Each of these factors is evaluated in the light of ethical conduct.
- Modifications or terminations: the IRB can require changes to a trial's protocol, consent process, or other trial aspects before the trial starts. They can also stop trials if they believe the patient's rights or welfare are at risk.
- Regulatory compliance: the IRB’s oversight ensures that trials comply with federal regulations governing human subjects in clinical research.
Additionally, throughout the trial, the IRB remains apprised of any issues that may affect patients, such as protocol changes or adverse effects.
Institutional Review Board (IRB) Approval For Recruitment
Despite their good intentions, IRBs can create roadblocks to patient recruitment. First, when sponsors are launching multi-site trials, they must meet the criteria for disparate local IRBs. When these criteria are at odds with each other, and the study is approved in some locations but not others, the study launch can be slowed or halted. This makes consistent, comprehensive patient recruitment challenging.
Secondly, meeting IRB approval creates a significant administrative burden for researchers. Also, local IRBs may have limited experience with novel trial designs, such as decentralized clinical trials (DCTs), leading them to request changes that actually place more burdens on patients, making recruitment and enrollment more difficult. However, early planning and open communication between sponsors, researchers, and IRBs help mitigate the delays that the difficulties in meeting IRB approval can cause.
Engaging Physicians To Support Recruitment Efforts
If you talk to someone who participated in a clinical trial, their story often begins, “I heard about the study from my doctor.” Physicians are usually seen as gatekeepers for clinical research, recommending eligible patients who could benefit from the trial. However, physicians remain underinformed regarding clinical trial opportunities and are hesitant to recommend them due to a lack of professional or financial benefit. Sponsors must intentionally appeal to doctors and physicians in addition to directly marketing to patients to improve their recruitment rates.
Preparing For Patient Recruitment
Participants are more likely to sign up for clinical trials that are beneficial and feasible. Study design and site selection are two factors that can affect enrollment, but a good strategy begins with understanding the targeted patient population.
Importance Of Early Planning For Recruitment Success
Recruitment and enrollment can create bottlenecks and are often the lengthiest steps in a clinical trial. Planning can save sponsors valuable time and prevent stalled studies. Delayed recruitment leads to higher costs, longer timelines, and a risk of losing the opportunity to research life-changing medicine.
Planning entails several vital aspects:
- Research the patient population’s needs and barriers to participation.
- Design recruitment materials tailored to the patients’ perspectives.
- Demonstrate to the patient how the study can benefit them.
- Engage healthcare providers and patient advocates.
- Leverage multi-channel marketing strategies.
- Optimize site selection and feasibility.
- Strategize for patient retention.
Effective Protocol Design For Recruitment Success
Study designers must consider the needs and constraints of their targeted population rather than merely focusing on scientific concerns. For example, when a study involves minor children, study designers should consider the needs of parents and caregivers as well as the children participating in the trial.
Defining Inclusion And Exclusion Criteria
Inclusion and exclusion criteria define the clinical trial’s target population. These criteria must be carefully designed to ensure the study population is appropriate for answering the research question while minimizing the risk of bias and creating generalizable results. When criteria are too narrow, patient recruitment is severely restricted, making it more challenging to maintain study timelines and budgets. When determining inclusion and exclusion criteria, researchers must balance scientific needs with the reality of study recruitment.
Establishing Study Endpoints And Outcomes
Endpoints and outcomes directly influence a trial’s design, sample size, and statistical relevance. When endpoints are clinically meaningful and relevant, participants are more likely to enroll in the study. Conversely, endpoints and outcomes that are poorly defined, difficult to quantify, or don’t directly benefit patients can hinder recruitment.
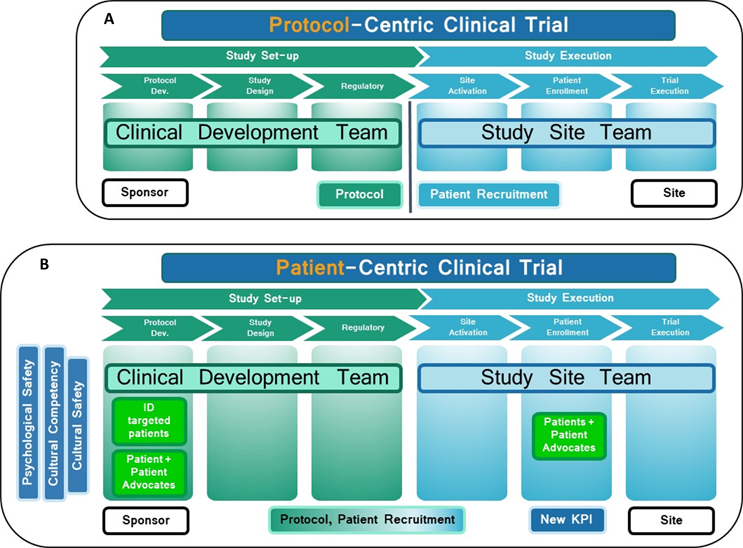
Source: How Psychological And Cultural Safety Can Accelerate Diverse Patient Recruitment And Retention, Valerie Schaeffer, 2022
Strategic Site Selection And Evaluation
Choosing suitable sites for clinical research has profound implications for patient recruitment. Even if the study uses DCT elements, clinical research sites can make or break a study's success. Well-selected sites accelerate enrollment, while poor selection leads to under-enrolled, delayed, and overbudget trials. Thus, sponsors must choose sites carefully and prioritize their relationships with study coordinators and PIs.
Conducting A Comprehensive Site Feasibility Assessment
Sponsors benefit from conducting a feasibility assessment on potential sites. While cost is a limiting factor, sponsors should also ask the following questions:
- Where is the site located? Common sense dictates that successful sites are located in large urban areas to reach the most patients. However, this approach excludes patients from rural areas. Instead, sponsors should cultivate a mix of site locations to ensure diverse recruitment.
- Does the site contain the necessary equipment and facilities for the trial? Depending on the trial’s protocol, sites may need specialized lab equipment, such as imaging devices, or have access to labs and facilities to provide these services. If sites are shipping samples to external labs, then they need access to couriers or shipping centers. Sites also need secure file storage and a data management system that collects and transfers sensitive clinical trial data.
- What is the site’s experience with similar trials? For example, if a sponsor wants to run an oncology trial, choosing a site with previous oncology experience helps ensure the site has the staff and equipment required for the study. When sites are familiar with the therapeutic area, they can launch studies more quickly, too.
- Are the PIs and sub-investigators up-to-date on licensing requirements? Validating investigators' licensure up front saves regulatory headaches down the road.
- Can the site meet regulatory approval? Sites must prove they can meet Good Clinical Practice (GCP) standards for designing, conducting, recording, and reporting trials. They also need to have IRB approval and quality control systems while still meeting other study-specific requirements for regulatory approval before the trial begins.
- Does the site have the necessary bandwidth for your trial? Competition and study load are further limiting factors. Sites conduct multiple studies, and sponsors should realistically consider whether a site has the capacity to take on new studies.
Investigator And Site Staff Training For Recruitment Efficiency
Sponsors are legally required to select qualified PIs to lead clinical studies who have at least two years of experience in clinical research and GCPs. The PI, in turn, is responsible for selecting, vetting, and training site staff. When the PI is not a physician, sponsors must provide a qualified physician or dentist as a sub-investigator who assumes responsibility for trial-related medical decisions.
In addition to these requirements, sponsors must provide PIs and their subordinates with all necessary training materials for the initial protocol and throughout the study if the protocol changes. This includes all trial procedures, safe IP handling, and documentation. Training can be conducted remotely or in person as long as it meets these criteria.
Creating Compelling Recruitment Materials
Sites are also key to patient recruitment, as they maintain databases of potential participants that sponsors can use to assess feasibility and create strategies. Sponsors should provide recruitment material to sites and other medical providers to market the study directly to participants.
Designing Engaging Patient Information Sheets, Brochures, And Outreach Materials
While informational materials are subject to regulation, they must also engage patients with attractive designs and relatable content. When designing these materials, sponsors should use the following strategies:
- identify the target audience
- design attractive, informational marketing materials
- create information sheets.
Using Attractive And Informative Design
Effective materials should tell participants how they could benefit from the study while clearly communicating possible risks. Studies need participants to enroll and complete them. Communicating benefits and risks up front helps with both enrollment and retention.
Highlighting Study Benefits And Risks For Participants
Clear communication builds trust, and when recruitment materials plainly state the benefits and risks, participants can make informed decisions, improving retention rates.
Benefits
- Emphasize the high-quality care participants will receive throughout the study, including tests, labs, and doctor visits.
- Highlight access to cutting-edge treatments or therapies.
- Describe how the study may help future patients.
Risks
- Detail potential risks in clear, easy-to-read language.
- Explain how risks are monitored and managed during the trial.
Creating Clear And Understandable Informed Consent Forms
Consent forms must be accessible and comprehensible to participants to engender fully informed decision-making.
Ensuring Clarity And Addressing Potential Participant Concerns
First, consent forms should use plain language written at an 8th-grade reading level or lower. Whenever possible, the materials should avoid technical jargon and complex medical terminology. Any acronyms or abbreviations should be explained to avoid confusion.
Forms must also be well-organized and use visually compelling techniques such as tables and visual aids to explain complex concepts. Text should be broken up into scannable headings and subheadings, emphasizing essential points with bold text or text boxes. Importantly, materials should be presented in the language native to the participant to facilitate understanding.
Consent forms must also include:
- The purpose of the research,
- Expected duration,
- Description of procedures,
- Potential risks and benefits, and
- Alternative treatments available.
IRBs review consent forms for compliance, but sponsors also benefit from conducting focus groups with potential participants to test the forms' readability.
Developing Effective Advertising And Outreach Strategies
Advertising for clinical trials entails multi-pronged patient outreach strategies, including digital and print materials and direct interactions with patients, advocacy groups, and healthcare providers.
Leveraging Digital Recruitment Channels
Digital communities, such as social media groups, can be a treasure trove of recruitment opportunities when fully optimized. However, sponsors need to design their digital strategies carefully to avoid marketing to the wrong populations or failing to reach their target audience.
Social Media Marketing
Social media spaces such as Facebook, Instagram, or TikTok offer free platforms that sponsors can leverage to reach their desired audience. Even paid advertising is financially feasible compared to traditional advertising channels. Campaigning on social media has the potential to reach a large audience with specific health conditions and gives online communities, such as patient advocacy or disease support groups, the ability to share trial information. Additionally, participants can use fillable forms to contact sponsors or engage with pre-screening quizzes.
Online Patient Communities
Individuals and families managing serious diseases often join online support communities to share resources and experiences. Sponsors should research the communities available to their target population and engage directly with community leaders to share information about the study.
Search Engine Optimization (SEO)
Another way for sponsors to increase their trial's visibility is to enhance the study's website via SEO to rank high on search results and reach a larger audience. Keyword optimization, content marketing, and link building are a few methods designers can use to improve SEO.
Engaging Communities Through Outreach Programs
The digital world is immense, but it does not reach everyone. It’s important to remember that not all patient populations spend time online. Digital literacy and technological access are two barriers that might keep participants from learning about a study. Therefore, sponsors need on-the-ground methods, too.
Local Health Fairs And Events
Events focused on a specific disease or disease area, such as rare diseases or cancers, can be great opportunities to advertise clinical trials. Representatives can directly connect with potential participants, share materials, and answer questions. This face-to-face interaction can also help dispel myths and misconceptions about trial participation. Sharing success stories at these events is also advisable to further humanize the experience.
Collaboration With Healthcare Providers
The key to encouraging healthcare providers (HCPs) to recruit subjects is to show them how it would benefit their patients. An HCP's first duty is to protect patient health; sponsors must show how their trials put participants first and offer them valuable opportunities. Providing HCPs with easy-to-use, accessible materials also encourages them to spread the word. Sponsors who have already established relationships with HCPs can leverage those connections to share information about upcoming studies.
Strategies For Patient Engagement
“Patient centricity” may be a buzzword, but at its core, it asks sponsors to empathize with patients and build trials around their needs rather than asking patients to build their lives around the clinical trial. Leading with empathy for participants affects protocol design, clinical trial technologies (e.g., DCT capabilities), and site selection, among other trial aspects. Although patient-centered designs require more up front planning, they make recruiting and retaining participants easier for sponsors.
Implementing A Patient-Centric Approach In Recruitment
First, sponsors and study designers need to understand the unique needs and concerns endemic to their target population. Fortunately, there are concrete steps that make this process easier and more effective.
Collaborating With Patient Advocacy Groups
Patient advocacy groups provide a platform where patients are the experts on their condition. This allows sponsors to better understand the patient's perspective and design more patient-centric trials. Collaborating with these groups can help build trust and confidence within the patient community, making them more willing to participate in clinical trials.
Engaging patient advocacy groups as advisors throughout the trial design and execution process ensures the trial is optimized for patient participation and retention. This includes providing feedback on protocol, endpoints, and patient-facing materials.
Additionally, patient advocacy groups can leverage their communication channels and networks to spread awareness about specific clinical trial opportunities, helping sponsors reach and recruit the target patient population. They can also help identify and connect sponsors with key opinion leaders and patient influencers who can promote trial awareness and recruitment within their communities.
Empowering Patients Through Education And Engagement
Establishing relationships with patient advocacy groups is the first step in educating and powering patients. But sponsors also need a plan to communicate with patients directly and openly to encourage enrollment. First, a dedicated website with robust search functionality makes it easy for patients to share information through social media.
Addressing Common Misconceptions And Stigmas Around Clinical Trials
Common misconceptions about clinical trial participation include the following: clinical trials are dangerous, provide no benefit to the participant, are only “last resort” options, or are time-consuming or expensive. Through advocacy groups and other campaigns, sponsors need to educate patients about the benefits of participating in clinical research while assuring them that ethical and medical oversight protect their safety. Adopting patient-centered approaches, such as building feasible protocols and reimbursing participants for their time, improves enrollment.
Likewise, patients struggle with the stigmas that may be attached to clinical trials. For example, if people are embarrassed to report their disorder to their doctor, such as those struggling with substance abuse, they are less likely to participate. When dealing with sensitive subjects that carry social stigmas, researchers must first set aside their prejudices and misconceptions to avoid alienating participants. Sponsors should also ensure that their recruiting and informational materials avoid stigmatizing language and present information in an unbiased, scientific way. Sponsors who engage with community partners and advocate for patients dealing with socially disadvantageous diseases and conditions also earn patients' trust.
Building Trust And Transparency With Participants
Ongoing misconceptions about pharmaceutical companies and clinical trials have eroded trust in many communities. To combat this, sponsors should provide data transparency, sharing the results of previous and ongoing trials to establish credibility. Throughout the recruitment process, sponsors should present complicated health information in an easily understandable way to address language or cultural barriers, especially for underrepresented minorities. Improving patients’ health literacy through clear, reliable communication and materials builds trust and rapport with participants.
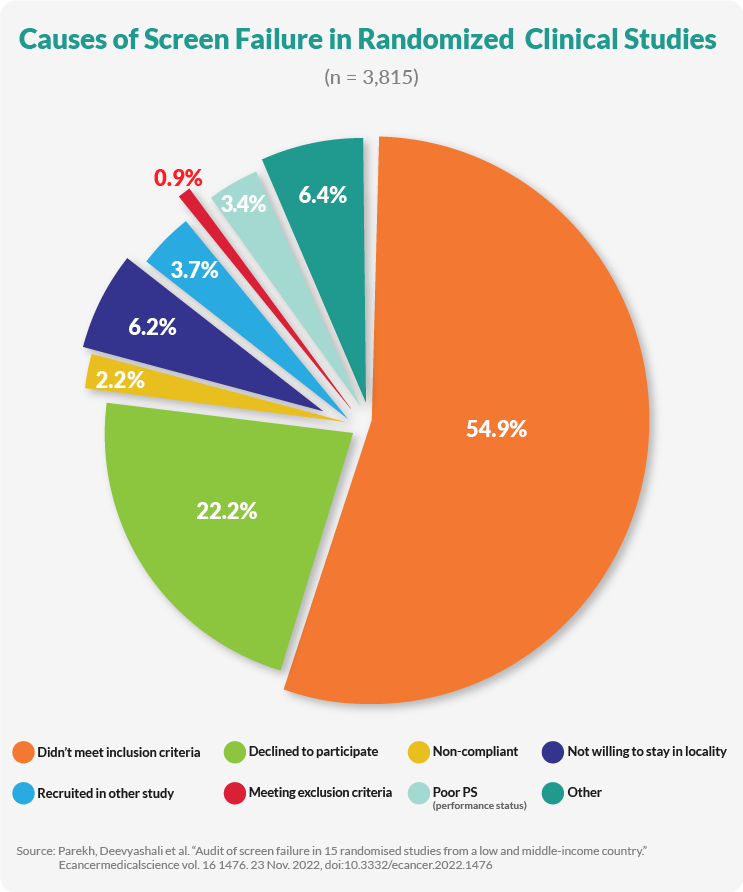
Simplifying The Screening Process For Faster Enrollment
Recruitment is but the first step in the enrollment process. Once participants are recruited, they are screened, and unfortunately, only 27% of volunteers screened meet the requirements for participation, and attrition rates continue to be high. To compensate for these numbers, sponsors are increasing their recruitment budgets. However, simplifying the screening process can help sponsors save time and labor costs while reducing attrition.
You can improve enrollment rates by making screening easier for participants, such as by optimizing the number of times interested participants have to visit a clinical site for baseline tests. Also, targeting specific populations, rather than trying to cast the widest net possible, improves enrollment rates by reducing the number of participants who fail screening. Pre-screening tools, such as online screening surveys linked to social media ads, help identify the best candidates ahead of time, saving site staff untold hours in wasted screening tests. Additionally, the following approaches can streamline the process:
- Implement pre-screening tools.
- Enhance communication with potential and enrolled participants.
- Use technology to make participation easier.
- Focus on patient-centric approaches.
- Optimize site and staff resources.
- Adopt data-based recruitment strategies.
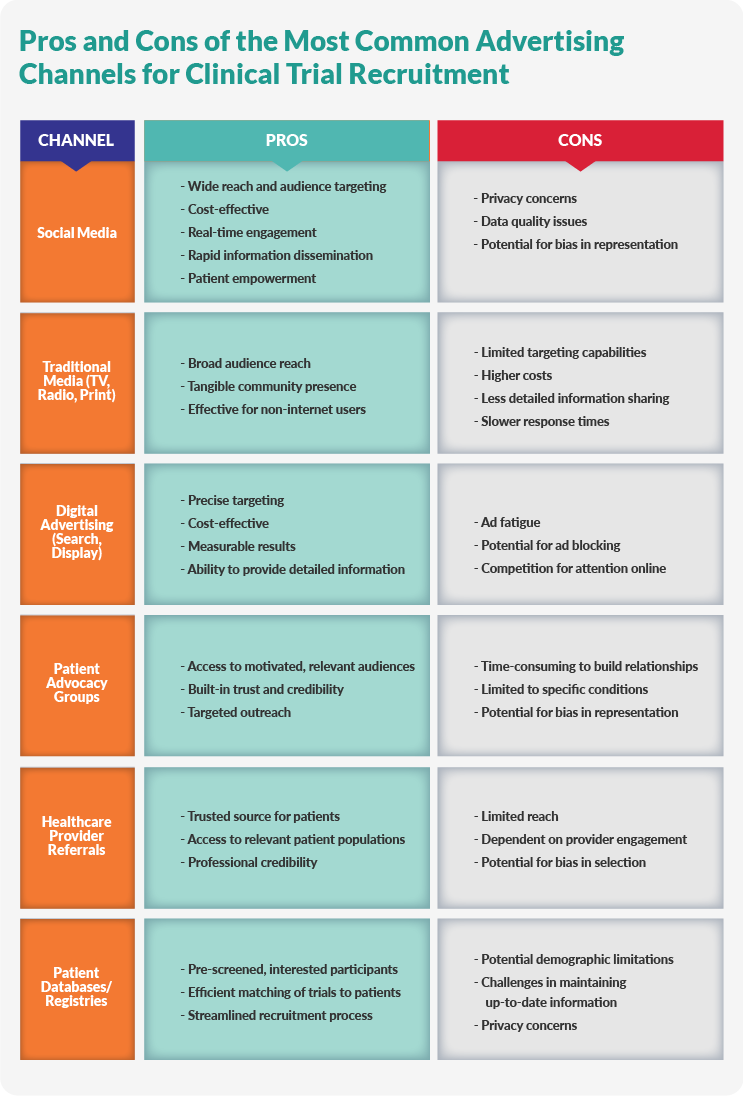
Utilizing Digital Recruitment Channels For Broader Reach
Digital communities, e.g. social media groups, can be a treasure trove of recruitment opportunities when fully optimized. However, sponsors need to design their digital strategies carefully to avoid marketing to the wrong populations or failing to reach their target audience.
Leveraging Social Media For Clinical Trial Recruitment
Social media spaces such as Facebook, Instagram, or TikTok offer free platforms that sponsors can leverage to reach their desired audience. Even paid advertising is financially feasible compared to traditional advertising channels. Campaigning on social media has the potential to reach a large audience with specific health conditions and gives online communities, such as patient advocacy or disease support groups, the ability to share trial information. Additionally, participants can use fillable forms to contact sponsors or engage with pre-screening quizzes.
Engaging Patients Through Online Communities
Individuals and families managing serious diseases often join online support communities to share resources and experiences. Sponsors should research the communities available to their target patient population and engage directly with community leaders to share information about the study.
Search Engine Optimization (SEO) For Patient Recruitment
Another way for sponsors to increase their trial’s visibility is to enhance the trial’s website via SEO. When the trial’s website uses SEO strategies effectively, it will rank high on search results and reach a larger audience. Keyword optimization, content marketing, and link building are a few methods that designers can use to improve SEO.
Maximizing Community Outreach Programs
The digital world is immense, but it does not reach everyone. It’s important to remember that not all patient populations spend time online. Digital literacy and technological access are two barriers that might keep participants from learning about a study. Therefore, sponsors need on-the-ground methods, too.
Participating In Local Health Fairs And Events
Events such as those focused on a specific disease or disease area, such as rare diseases or cancers, can be great opportunities to advertise clinical trials. Representatives can directly connect with potential participants, share materials, and answer questions. This face-to-face interaction can also help dispel myths and misconceptions about trial participation. It is also advisable to share patient success stories at these events to further humanize the experience.
Collaborating With Healthcare Providers For Recruitment
The key to encouraging healthcare providers (HCPs) to recruit patients for clinical trials is to show them how the trial would benefit their patients. An HCP’s first duty is to protect patient health; sponsors need to show how their trials put patients first and offer them valuable opportunities. Providing HCPs with easy to use, accessible materials also encourages them to spread the word. Also, if sponsors have already established relationships with HCPs, they can leverage those connections to share information about upcoming studies.
Collaborating With Research Sites For Recruitment
Sponsors can take actionable steps to improve clinical trial site collaboration, such as:
Provide Comprehensive Support
Supply recruitment materials, tools, and resources to sites and provide training on recruitment best practices and protocol-specific strategies.
Establish Clear Communication Channels
Communication between sponsors and sites is crucial. This includes regular check-ins to discuss recruitment progress and challenges and a system in which sites can easily reach out with questions or concerns. Sponsors should also provide transparency by sharing recruitment updates and successes across all participating sites.
Leverage Site Expertise
Effective sites have their own recruitment strategies, so sponsors should encourage them to share their local knowledge and successful tactics and give sites the flexibility to tailor recruitment approaches to their specific patient populations.
Set Realistic Goals And Incentives
Goal setting should be realistic, and sponsors should work with sites to set achievable targets. Sponsors should also offer incentives for meeting or exceeding recruitment goals and recognize and reward high-performing sites.
Enhance Site Capabilities
Technological solutions can streamline recruitment by providing database mining and patient identification. Sponsors should provide technology that assists sites in optimizing their internal processes for efficient screening and enrollment.
Foster A Collaborative Environment
Collaboration between participating sites improves recruitment. Meetings or workshops where sites can share experiences and best practices foster a sense of community across sites, and a little friendly competition can boost recruitment efforts.
Address Site-Specific Challenges
Sites that face challenges need additional support. Sponsors should provide resources to underperforming sites and listen to site feedback to adjust strategies as needed.
Enhancing Patient Experience During Enrollment
Given the disparity between recruitment numbers and enrollment eligibility, qualified participants should be treated like the clinical trial MVPs that they are. When sponsors adopt a patient-first approach to trial design, they have a higher chance of keeping these MVPs on the team for the duration of the study.
Optimizing Screening Processes For Better Patient Experiences
An efficient and patient-friendly screening process can improve recruitment rates and trial success.
Streamline Screening Procedures
Screening procedures should not place undue burdens on potential participants. For instance, electronic health records can be used to pre-identify potentially eligible patients, allowing for a more efficient screening process. Additionally, the required screening visits should be minimized, with initial pre-screenings conducted remotely when possible.
Improve Communication
Potential participants should be given clear information about screening requirements up front. Sponsors should provide multiple communication channels (e.g., phone, email, and text) for patient questions and send reminders about upcoming screening appointments.
Enhance Convenience
Prioritizing patient-centricity in screening means offering flexible scheduling options, providing transportation assistance when needed, and considering mobile or at-home screening for certain assessments.
Use Technology Effectively
Electronic consent forms, apps, and portals can simplify screening processes and provide a more user-friendly experience. Some screening procedures can be conducted remotely to provide further convenience.
Personalize The Experience
Staff should be trained in patient-centered communication. This includes allowing sufficient time for patients to ask questions and voice concerns and providing a single point of contact to guide patients through screening.
Minimize Patient Burden
Look for ways to reduce patient burden as much as possible. For example, combining screening procedures means fewer required visits. To safeguard patients' time, only essential data should be collected during screening, and sponsors can offer compensation for their time and travel expenses.
Provide Timely Follow-Up
Patients should not wait long to find out if they are eligible to participate. Screening results should be shared promptly, with clear next steps for eligible and ineligible patients. Ineligible patients should be given referrals or other options for care.
Offering Comprehensive Patient Support Services
Sponsors and study designers should ask themselves, how can we make this trial less burdensome and more convenient for the participants? This question entails considering the participants' lives outside of the study and includes their caregivers' needs when appropriate.
Providing Transportation Assistance To Reduce Participant Burden
How easy is it for participants to visit clinical sites? Are they traversing long distances or spending money on transportation? Do they need caregivers to drive or escort them to sites?
Ideally, clinical sites are centrally located and accessible to patients, but the reality is many patients live in rural areas, far from major academic or health centers. If the sites aren't convenient to patients, consider incorporating DCT elements to reduce site visits, such as telehealth capabilities or home-based nursing services. Also, providing transportation assistance or reimbursement can ease patient burden. Finally, consider scheduling clinical visits outside of regular working hours, such as evenings and weekends, so that patients don't have to take time off of work to participate.
Addressing Language And Cultural Considerations In Recruitment
Are your materials presented in your targeted population’s language? Are they culturally sensitive? Do you provide interpreters at sites, if appropriate? When communication breaks down, trust erodes, and participants drop out of studies. Consent forms, educational materials, and site staff need to be accessible and meet patients where they are.
Maintaining Clear Communication And Setting Proper Expectations
High dropout rates are unfortunately common in clinical trials, but good communication strategies help ensure participants are fully knowledgeable and committed before the study begins.
Outlining Study Procedures, Timelines, And Commitment
No one likes a bait-and-switch situation. When they commit to clinical research, participants want to understand the study's procedures and timelines in depth. If the procedures are unclear, patients may encounter unpleasant surprises and drop out. Likewise, if the study timeline is vague, participants may hesitate to sign up for an open-ended commitment. Establishing these parameters up front aids in enrollment and retention.
Clarifying Potential Risks And Benefits For Participants
Participants are more likely to enroll when participation is framed as care. Even if the patient receives a placebo, they will receive excellent care throughout the study through tests, labs, and doctor visits. Emphasize to participants that during the trial, they will receive best-in-class healthcare.
However, participants should also be aware of the risks they may encounter during the trial. Informed consent is legally required, but sponsors should go above and beyond regulatory requirements to thoroughly explain risks to patients. Clarity and openness build trust and reduce attrition rates.
Adapting Recruitment Strategies Based On Participant Feedback
Finally, listen to patients. During the recruitment campaign, invite feedback from potential participants through surveys and other interactive tools and look for patterns. Why do people hesitate to enroll? Is it the time burden? Do they fail to see a clear benefit to themselves? Are they afraid of the risks? Feedback at this stage is invaluable to sponsors who can tweak their messaging or offer patients more participation options before a study begins.
Monitoring And Evaluation Of Recruitment Efforts
In addition to participant feedback, sponsors benefit from leveraging data to monitor and evaluate their recruitment efforts continuously.
Tracking Key Recruitment Metrics For Continuous Improvement
Continuous data analysis allows sponsors to track the following and take actionable steps:
Measuring Screening And Enrollment Rates
Sponsors should track the percentage of participants screened successfully and enrolled in the study, the cost per qualified patient, enrollment rates across multiple sites, reasons for screening failures, and patient demographics. These metrics are crucial to evaluating the cost effectiveness of a trial's outreach campaign.
Analyzing Conversion Rates At Different Recruitment Stages
Conversion numbers are more complex than merely calculating the percentage of applicants who enroll. Each stage of the recruitment process should be tracked, from initial interest to pre-screenings to screenings and enrollment.
Identifying And Addressing Areas For Improvement
When examining the above, sponsors can identify root problems and find solutions. For example, if high numbers of individuals fail pre-screenings, then recruitment efforts are casting too broad of a net and need to be narrowed. If patient demographics fail to reach diversity targets, sponsors can consider alternative methods to reach marginalized populations.
Data-Driven Decision-Making In Recruitment
Data can inform each stage of the recruitment process and help sponsors navigate the following:
Identifying Underperforming Sites
Sponsors use data to identify underperforming sites to determine the next steps. Some sites may need more materials and support from sponsors, while others might not be a good fit for the trial due to factors such as inexperience in the therapeutic area or location. Identifying underperforming sites early on and determining whether their problems are fixable can save sponsors wasted time later on.
Adapting Recruitment Strategies To Meet Trial Goals
Adopting a continuous improvement mindset throughout recruitment can help sponsors meet enrollment and timeline goals.
Ensuring Flexibility In Recruitment Approaches
As the recruitment timeline progresses, staying flexible and changing the approach when needed in response to recruitment data is essential. Being open to new strategies as well as willing to abandon ineffective ones leads to a more successful campaign. Once the underlying issue is determined, sponsors can find creative solutions to enrollment challenges.
Addressing And Overcoming Unforeseen Challenges
Common recruitment challenges and solutions include:
- Lack of Diversity: If sponsors struggle to meet their demographic goals, they need to examine whether the study presents barriers to participation for marginalized groups. For instance, certain groups may need alternative hours for site visits or assistance with transportation. Engaging with patient advocacy groups can help sponsors tailor studies to underrepresented populations.
- Participant drop out: If potential participants initiate the screening process but then drop out, sponsors may need to reconsider their data-collection methods. Offering participants a variety of options, from in-person visits to mailed-in kits to online surveys or phone interviews, helps participants feel engaged with the screening process and allows them to choose a method that is comfortable and convenient for them.

Retention Strategies For Participants
Successful clinical trials also must retain as many enrolled participants as possible to meet their study and timeline goals and collect valuable long-term follow-up data. By making participation as convenient and rewarding as possible while showing ongoing appreciation for participants’ contributions, sponsors can establish a positive connection with subjects that keeps them invested in the trial.
Retention Solutions For Maintaining Participant Engagement
The first step in keeping participants engaged is ensuring they fully understand the study requirements and time commitment up front. Patient retention strategies in clinical trials also include providing flexible scheduling options for study visits, appointment reminders, transportation assistance or reimbursement, and compensation.
Combining study procedures when possible can minimize the participant burden. Additionally, the site should create a welcoming and comfortable environment with a staff fully trained in patient-centered communication. Participants should have multiple channels to reach the study team, such as email, portals, phone numbers, and text support. When appropriate, sponsors should share updates and interim results to show participants how they contribute to the study.
Long-Term Patient Engagement And Follow-Up
Clinical trials frequently require long-term engagement. To keep participants engaged over time, sponsors should develop a personalized follow-up plan for each individual. Periodic newsletters or updates about the study's progress remind participants of the study's importance. Patient portals or mobile apps and regular patient check-ins via phone, texts, or emails between visits keep patients engaged.
Long-term follow-up visits are often performed remotely, which can be more convenient for participants. Milestones and participant contributions should be acknowledged, and events or support groups for study participants maintain their connection to the study. Finally, throughout the trial, sponsors should demonstrate gratitude and emphasize the value of their continued participation.
Regulatory And Ethical Considerations
For the last several decades, national and international regulatory bodies have been working to improve and enforce human rights for subjects engaged in clinical trials. Good clinical practice (GCP) is an internationally recognized guideline for how clinical trials are conducted, implemented, monitored, audited, analyzed, recorded, and reported. Trials must be scientifically sound to prove a drug or device’s safety and effectiveness, but they also must be conducted humanely.
Regulatory bodies such as the FDA, EMA, ICH, and other national and regional organizations require companies to comply with GCP standards and other criteria before approval. These criteria include the following factors:
Ensuring Compliance With Regulatory Requirements In Recruitment
Sponsors must meet several criteria to meet GCP and other regulations, including:
- Informed Consent: Potential participants must be fully informed about the trial's purpose, risks, and benefits and give voluntary consent to participate. The informed consent process is critical to ensure participants understand what they are agreeing to.
- Fair Subject Selection: Participants should be selected based on the scientific goals of the study, not factors unrelated to the research. Exclusion of certain groups requires justification.
- Favorable Risk-Benefit Ratio: Researchers must minimize risks to participants and ensure the potential benefits outweigh the risks.
- Independent Review: An IRB should evaluate the ethical acceptability of the trial design and ensure protections for participants.
- Protecting Participant Rights And Safety: Participants' privacy, right to withdraw, and welfare must be respected throughout the trial. Researchers should communicate new information that may affect participants' willingness to continue.
- Recruitment Strategies: Recruitment methods like advertisements must be approved by the IRB to ensure they are not coercive or misleading.
- Participant Burden: The trial protocol should minimize the burden on participants through reasonable eligibility criteria, data collection schedules, and compensation.
- Monitoring And Oversight: Ongoing monitoring of recruitment progress, screening rates, and retention is crucial to identify and address any issues.
Maximizing Participant Diversity
Historically, women and minorities have been underrepresented in clinical trials, leading to a lack of understanding about the safety and efficacy of certain therapeutics on these populations. To improve clinical trial science, the FDA has repeatedly revised its regulations to ensure that studies are conducted on participants who accurately represent the targeted demographics of the therapeutic itself. Additionally, the FDA has released standardized diversity and inclusion language that companies can use to recruit participants for clinical trials to avoid confusion. Regulatory guidance covering diversity in U.S.-based clinical trials includes:
- The Consolidated Appropriations Act 2023, which includes the Food and Drug Omnibus Reform Act, requires sponsors of specific clinical trials to submit a Diversity Action Plan to the FDA. This plan must outline goals for enrolling underrepresented racial and ethnic participants.
- FDA guidance documents emphasize the importance of ensuring clinical trial populations represent the patients who will ultimately use the medical product. This includes direction on enhancing the diversity of clinical trial populations.
- NIH policy requires NIH-funded clinical trials to report on the inclusion of women and minorities.
- FDASIA Section 907 details the requirements for reporting demographic subgroup data in clinical trials.
Addressing Ethical Considerations In Patient Recruitment
Further ethical issues that sponsors must consider are:
Avoiding Coercion And Ensuring Ethical Recruitment Practices
Encouraging a person to participate in a clinical trial and coercing them are, naturally, not the same thing. Regulations are designed to ensure that the differences between those two methods are clearly and universally defined and enforced. The key regulations prohibiting coercion and undue influence in clinical trial recruitment are:
- The Common Rule (45 CFR 46) under the U.S. Department of Health and Human Services explains that informed consent be obtained "without coercion or undue influence."
- Additional protections lie under Subpart C of the Common Rule for research involving prisoners, who are considered a vulnerable population susceptible to coercion due to their confined and controlled environment.
- Guidance from the FDA and Office for Human Research Protections (OHRP) defines coercion as an "overt or implicit threat of harm" and undue influence as "an offer of an excessive or inappropriate reward" to obtain compliance.
- Requirements for IRBs to carefully review recruitment methods and informed consent processes minimize the possibility of coercion or undue influence, especially for vulnerable populations.
Finally, the FDA offers recommendations for study teams to design the consent process to reduce perceived pressure, such as having an independent party obtain consent, providing alternative options, and maintaining participant anonymity.
Upholding Confidentiality And Privacy In Clinical Trials
Participants’ confidentiality and privacy must be maintained throughout the clinical trial process. Regulations protecting these fundamental rights include:
- The Health Insurance Portability and Accountability Act (HIPAA) in the United States mandates privacy protections for individually identifiable health information collected during clinical trials. This includes obtaining patient authorization for use and disclosure of protected health information.
- The Common Rule (45 CFR 46) under the U.S. Department of Health and Human Services requires that informed consent processes address the extent to which participants' records will be kept confidential and gives guidance from regulatory bodies like the FDA and OHRP on best practices for maintaining participant privacy, such as secure storage of records, limiting access, and destroying data after the trial.
- IRBs must review and approve the confidentiality and privacy protections in clinical trial protocols before the research can proceed.
Additional state-level laws and regulations may further protect the confidentiality and privacy of clinical trial participants' information.
Leveraging Technology And Tools In Patient Recruitment
Recruiting patients for clinical trials is increasingly a high-tech enterprise. When optimized, these tools can shorten the recruitment process, create more targeted campaigns to reach specific populations, and increase success rates.
Harnessing The Power Of Electronic Health Records (EHRs) For Recruitment
EHRs can rapidly identify potential trial participants by screening patient data for specific eligibility criteria. Approximately 50% of site investigators search EHRs for possible subjects, often in conjunction with other recruitment methods like reviewing clinic schedules and contacting past trial participants. EHR-based recruitment can be less time-consuming and expensive than manual chart review and can help increase the number of eligible patients identified.
AI and machine learning are used to comb EHRs for relevant data, and one study showed an improved enrollment rate from 0.17 to 0.32 patients per screening day when using AI to mine EHR data.
Using Specialized Patient Recruitment Platforms
Additional specialized software tools like Patient Recruitment Systems (PRS) and Clinical Trial Recruitment Support Systems (CTRSS) can automate patient identification and screening using EHR data. These platforms use natural language processing to translate trial eligibility criteria into database queries, helping to find matching patient candidates.
Employing Data Analytics For Targeted Recruitment
Emerging AI technologies use large language models to assess whether a patient fits the criteria of a clinical trial. Also, digital twin technology creates digital simulations of patients, allowing researchers to predict patient outcomes. Other analytics tools summarize data from existing trials to help designers quickly understand how other trials have been designed and the outcomes.
Future Trends And Innovations In Patient Recruitment
New and emerging innovations such as the following promise to streamline the recruitment process, offering greater efficiency, personalized outreach, and shorter timelines.
The Role Of Artificial Intelligence In Modern Recruitment
The AI revolution shows excellent promise in targeting and recruiting participants for clinical trials. Currently, sponsors are leveraging AI to recruit patients in several ways:
- Automate Patient Recruitment: AI can automate and expedite the patient recruitment process. Generative AI can quickly and efficiently analyze large datasets of patient data to identify potential patients who may be eligible for clinical trials.
- Personalizing Recruitment Messages: Generative AI can also personalize recruitment messages to potential patients, increasing the likelihood that they will respond to recruitment messages and participate in the clinical trial.
- Tracking Patient Progress: Beyond recruitment, AI can track patient progress in clinical trials. This helps ensure patients receive the care they need, and the trial is progressing as planned.
For example, Pfizer uses a generative AI chatbot to interact with potential patients, answer their questions, and generate personalized recruitment messages. Merck has developed an AI platform to track patient data and identify those at risk of dropping out. These approaches help sponsors improve the efficiency, diversity, and success of their patient recruitment efforts.
Personalized Recruitment Strategies
Recruiting patients for personalized medicine trials should begin with patient centricity. These trials often involve patients with serious conditions that require caregivers. Given the inherent complexity of these studies, sponsors must be creative and compassionate when designing their protocols and recruitment campaigns by employing techniques such as the following:
- DCTs: Home-based trials allow patients to participate without having to travel to clinical trial sites, improving access and patient wellbeing.
- "Just-In-Time" Site Activation: Activating clinical trial sites "just-in-time" when identifying eligible patients helps overcome challenges in recruiting patients with specific genetic or disease profiles that may take months to find.
- Real-World Data And Machine Learning: Using real-world data from sources like registries, combined with machine learning, helps identify and recruit patients who match the specific genetic or disease profiles being targeted in personalized medicine trials.
- Patient Experience Improvement: Enhancing the overall experience for trial participants, from personalized communication to providing feedback, improves recruitment and retention.
Collaboration And Data-Sharing Initiatives To Improve Recruitment Efficiency
Collaboration and data sharing can help sponsors and other stakeholders recruit patients for clinical trials. Sponsors can pool resources with organizations like WHO, the NIH, and patient groups to share patient-donated data from clinical trials. This data sharing can transform the trial process, improving the patient experience and accelerating the discovery and delivery of new therapies.
However, effective data sharing is complicated by the need to protect patient privacy. The changing landscape of national and international regulatory requirements also creates difficulties. Nevertheless, better collaboration among sponsors is a potential solution to recruitment challenges.
Selecting The Best Patient Recruitment Approach
Patient recruitment is a complex, lengthy, and costly process that nonetheless is essential to launching a clinical trial. Sponsors succeed when they plan ahead, engage community members, leverage technology, and remain flexible and open to changing tactics based on feedback and data.
For example, emerging technologies are exciting, but companies should not assume that high-tech equals better. Digital recruitment methods can be highly effective under the right circumstances but may skew towards specific demographics. Interestingly, a review of clinical trial recruitment methods from 2012-2022 found that social media campaigns tended to recruit female, white, non-Hispanic, and college-educated subjects, indicating potential biases in outreach.
Another study found Facebook ads led to only two enrolled participants, while traditional electronic recruitment methods were more cost-effective. However, the use of targeted ads showing people who reflected the target population outperformed other ad versions, suggesting digital ads can be optimized for specific demographics.
According to these studies, addressing the "digital divide" is essential to the future of clinical trials, as socioeconomic, gender and geographic barriers can limit access to digital recruitment platforms, potentially exacerbating disparities. Strategies like using audiovisual materials and community engagement may help reach underrepresented populations.
Ultimately, the best tactics can be summed up with one word: empathy. Recruitment strategies based on empathy that consider the trial from a patient's perspective will consistently have an edge over those that prioritize science or cost over people. Given the vast unmet need for life-changing treatments, patient care remains at the heart of ethical clinical trials, beginning with recruitment.
Frequently Asked Questions (FAQs)
Below are frequently asked questions regarding clinical trial recruitment and enrollment.
1. How do you recruit patients for clinical trials?
Strategies include creating awareness through media, engaging healthcare providers, using patient databases, and implementing targeted outreach campaigns.
3. What are the three challenges to patient recruitment in clinical trials?
Lack of awareness, complex study protocols, and sociocultural issues related to trial participation.
4. Why is it hard to recruit patients for clinical trials?
Limited awareness, strict eligibility criteria, fear of side effects, geographical constraints, and misconceptions about clinical trials make recruitment challenging.
5. What are some significant challenges in recruiting participants into clinical trials?
Overoptimistic recruitment estimates, narrow eligibility criteria, recruiter lack of engagement, and insufficient funding are major challenges.
6. What are the main reasons for low patient recruitment and retention in clinical trials?
Complex protocols, lack of awareness, sociocultural issues, fear of side effects, and geographical distance from study sites.
7. How much does recruiting a patient for a clinical trial cost?
The cost varies widely depending on the study but can range from thousands to tens of thousands of dollars per patient.
8. Who is responsible for clinical trial recruitment?
The responsibility is shared among sponsors, investigators, research coordinators, and sometimes specialized patient recruitment firms.
9. How much do pharma companies spend on patient recruitment?
Pharmaceutical companies spend billions annually on patient recruitment, but exact figures vary widely and are often not publicly disclosed.
10. What are the barriers to patient participation in clinical trials?
Barriers include lack of awareness, fear of side effects, complex protocols, geographical distance, and misconceptions about clinical trials.
11. How are patients recruited for clinical trials?
Patients are recruited through healthcare provider referrals, media campaigns, patient databases, community outreach, and online platforms.
12. What does a patient recruitment specialist do?
They develop and implement strategies to identify, engage, and enroll suitable participants for clinical trials, often using various marketing techniques.
13. How are patients selected for clinical trials?
Patients are selected based on the study protocol's specific inclusion and exclusion criteria, ensuring they meet all requirements.
14. How do people learn about clinical trials?
People learn about trials through healthcare providers, media, patient advocacy groups, online registries, and word-of-mouth.
15. How is recruitment reporting typically handled?
Recruitment progress is reported regularly to sponsors and ethics committees, often using standardized forms or electronic data capture systems.
References
- Addressing Demographic Disparities in Clinical Trials (hbr.org)
- Addressing stigma within the dissemination of research products to improve quality of care for pregnant and parenting people affected by substance use disorder - PMC (nih.gov)
- Clinical trial design in the era of precision medicine | Genome Medicine | Full Text (biomedcentral.com)
- Consent Process Best Practices for Special Populations to Reduce Perceived Pressure to Participate in Clinical Trials - ACRP (acrpnet.org)
- Digital approaches to enhancing community engagement in clinical trials | npj Digital Medicine (nature.com)
- Digital Marketing Tactics to Increase Diversity in Clinical Trial Recruitment (clarkstonconsulting.com)
- Ethics in Clinical Research: Foundations and Current Issues - Features | Wake Forest University School of Medicine (wakehealth.edu)
- Facilitators of Successful Inclusion in Clinical Research | Improving Representation in Clinical Trials and Research: Building Research Equity for Women and Underrepresented Groups | The National Academies Press
- Harnessing artificial intelligence to improve clinical trial design | Communications Medicine (nature.com)
- How is Privacy Maintained During Clinical Trials? - Clinical Research Center Of The Carolinas (clinicalresearchcarolinas.com)
- Human subjects in clinical trials: Ethical considerations and concerns (oatext.com)
- A novel approach to conducting clinical trials in the community setting: utilizing patient-driven platforms and social media to drive web-based patient recruitment | BMC Medical Research Methodology | Full Text (biomedcentral.com)
- Patient Advocates and Researchers as Partners in Cancer Research: A Winning Combination | American Society of Clinical Oncology Educational Book (ascopubs.org)
- Patient Recruitment in Clinical Trials: Steps to Develop a Successful Enrollment Strategy (ohsu.edu)
- Patient Recruitment System for Clinical Trials: Mixed Methods Study About Requirements at Ten University Hospitals - PMC (nih.gov)
- Patient Recruitment in Clinical Trials: Areas of Challenges and Success, a Practical Aspect at the Private Research Site (scirp.org)
- Sponsor-Investigator Training: Module 3 – Good Clinical Practice (GCP), Essential Documents, & IND Trial Master File (chop.edu)
- Top 5 Misconceptions About Clinical Trials | Renown Health
- Top 5 Tips for Collaborating with Patient Advocacy Groups for Your Patient Recruitment Efforts - ACRP (acrpnet.org)
- The use of electronic health records for recruitment in clinical trials: a mixed methods analysis of the Harmony Outcomes Electronic Health Record Ancillary Study | Trials | Full Text (biomedcentral.com)
About The Author
Elizabeth Mann is a skilled writer with over a decade of experience in content creation, specializing in the life sciences industry. As a writer for Life Science Connect, she develops in-depth content that informs and engages professionals in the pharmaceutical, biotechnology, and medical device sectors. Her areas of focus include biologic drug production (including cell and gene therapies), clinical trial design and execution, and drug development and manufacturing outsourcing.
EXPERT INSIGHTS ON PATIENT RECRUITMENT
-
The Problem With Excluding Children From GLP-1 Trials In The U.S.
In the U.S., roughly 15 million children are obese. But many are excluded from GLP-1 research. Obesity expert Evan Nadler, MD, explains why that's problematic.
-
How AI-Enabled Personal Health Tools Are Reshaping Clinical Trial Workflows
Learn how AI-enabled personal health technologies are impacting key aspects of trial operations while also exploring operational challenges that clinical operations leaders must manage in this AI-driven era.
-
4 Steps For Representative Enrollment In Rare Disease Trials
Discover the D.A.T.A. method and its utility in enrolling diverse representative patient populations in rare disease clinical trials.
-
FDA Issues Final Guidance On Clinical Trial Participation: What You Need To Do Now
On December 15, 2025, the FDA finalized its guidance Enhancing Participation in Clinical Trials, formally updating expectations for enrollment and trial design. Here's what you can do now.
-
What ChatGPT Ads Will Really Mean For Clinical Trials
AI health assistants may be used to surface clinical trials earlier in patient journeys, and advertising inside conversational AI is best understood as the next logical step in that same progression.
EDITORIAL PERSPECTIVES ON PATIENT RECRUITMENT
-
AI Trial Matching Comes Of Age At City Of Hope
City of Hope has embedded an internally trained AI platform into oncology care and research workflows, helping clinicians summarize complex patient histories and match patients to clinical trials in real time. By reducing manual review and accelerating feasibility assessments across its national network, the system is improving trial access, easing clinician workload, and shifting more time back to patient care.
-
Informed Consent Isn't Broken — But It's Barely Working
Former FDA Commissioner Dr. Robert Califf has been blunt about what’s wrong with informed consent in clinical trials. In this article, we offer a high-level look at his three-part Substack series, exploring how consent became more about legal protection than patient understanding — and what needs to change.
-
When Clinical Trials Don't Stop For War
When war halted clinical trials in Ukraine, sites adapted instead of shutting down. Dr. Anna Titkova explains how patients, investigators, and sponsors kept research moving when conditions were anything but stable.
-
The Case For Research-Naive PIs
Carrie Lewis, executive director, clinical program optimization, at Keenova (formerly Endo) explains why research-naive PIs may improve trial quality, challenge assumptions about ROI, and help fix a shrinking investigator pipeline.
-
Funding Diversity Where It Starts — At The Site
After crossing paths with retired family physician and researcher Dr. Bryce Palchick at two conferences, I learned about ACRO’s new Diversity & Inclusion Site Resource Grants Program — an effort funding community sites like Bryce’s to make trial diversity more than a buzzword by supporting real, grassroots engagement.
FREE DEI E-BOOK

Download this free Clinical Leader e-book to learn about the barriers to clinical trial participation, as well as how to develop a diversity action plan (DAP) and achieve success in improving diversity in trial design, recruitment, enrollment, retention, and reporting.










