Rare Disease vs. Chronic Conditions: Recruitment And Engagement Strategies
By Deborah Howe, director of global patient recruitment and engagement, Alexion, and Michele Teufel, senior director, TA strategy, & portfolio delivery, AstraZeneca

Rare disease and chronic conditions, when it comes to studying their interventions in clinical trials, have many commonalities as well as differences. Following a co-presentation at SCOPE 2023, this article explores the similarities and differences in recruitment and engagement strategies for rare diseases versus chronic conditions in the clinical trial setting.
A variety of approaches are discussed, including patient centricity and insights, protocol feasibility, centralized and localized patient recruitment and retention tactics, site support services, and more. The authors of this article bring different perspectives based on the clinical trials they oversee — specifically, the rare disease portfolio at Alexion Pharmaceuticals and chronic conditions (such as asthma, COPD, heart failure, and chronic kidney disease) in the AstraZeneca (AZ) portfolio.
What Is The Difference Between Rare Disease And Chronic Conditions?
Generally speaking, a rare disease is one that affects a small percentage of the population; however, the definition varies around the world. In the U.S., for example, a rare disease is defined by the Orphan Drug Act as a disease or condition that impacts fewer than 200,000 people1. In Japan, the legal definition of a rare disease is one that affects fewer than 50,000 patients in Japan, or about 1 in 2,500 people.2 Multiple sources, including the NIH, claim there are between 7,000 and 10,000 rare diseases in the world and, of these, only 5% have approved treatments.
In contrast, chronic conditions are defined as a disease or condition that is long lasting, for at least three months or more. For the purposes of this discussion, we are also referring to chronic conditions as non-rare in nature.
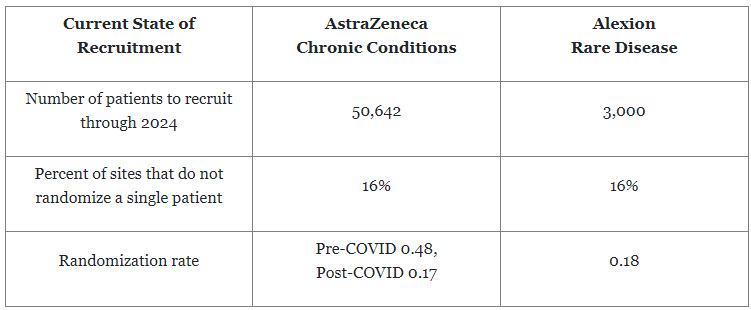
Differences In Patient Recruitment — Number And Enrollment Date
We thought it would be helpful to share the projected number of patients to be recruited across AZ’s chronic trials versus Alexion’s rare trials. We expected the AZ projected patient numbers would be higher, but we were not expecting the percentage of sites that don’t randomize a single patient to be the same.
For both types of conditions, an enormous amount of effort and cost are expended to identify, activate, and ensure sites recruit patients to deliver new medicines. Additional effort needs to be put into feasibility and site identification processes to ensure sites clearly understand the requirements for each study to minimize the number of sites not randomizing a single patient.
A point of distinction between recruiting for rare diseases versus chronic conditions is the randomization rates, defined as the number of patients recruited per investigative site per month. In the Alexion rare disease portfolio for 2022, the randomization rate ranged from 0.01 to 0.42 patients per site per month with an average of 0.18. For example, a typical rare disease study may activate 150 investigative sites to achieve a randomization goal of 254 patients, which means that each site would only be targeting to randomize 1 or 2 patients over the total randomization period. For chronic conditions, there has been a dramatic shift pre- and post-COVID. Before COVID-19, AZ’s chronic studies were randomizing approximately 0.48 patients per site per month; while post-COVID, it is randomizing 0.17 patients per site per month, which is currently in line with rare disease. However, AZ continues to work toward returning to pre-COVID rates.
Similarities In Site Engagement Strategies
We have collectively identified that early site engagement, site training, site engagement plans, and site support are common strategies across both rare disease and chronic conditions. The differences arise in the details, planning, and execution of these strategies.
It is critical to engage with our sites to gather their input as we develop our programs. Physicians contribute to the medicine and science, while study coordinators provide operational input. Both organizations strive to conduct study coordinator panels/discussions during program development, as this is an opportunity to gather key input on eligibility criteria, study visit schedule, and procedures from an operational standpoint. Study coordinators can also advise what might work well and where simple adjustments can minimize site and patient burden.
Our site partners are one of our key customers and training them effectively for each study is essential. We need to ensure training sessions provide a clear understanding of all endpoints and procedures —including why we are doing them. Once a study starts, it is essential to maintain an active dialogue with our sites. AZ typically holds open forums or hosts Q&A sessions once a month during the life of the study to maintain an active dialogue. Post-COVID, site resources have been very constrained, so we are working to identify various vendors to support enrollment, recruitment, data entry, travel support for patients, and reimbursement methods. Having a menu of support services will allow sites to pick and choose what they need for that specific study.
Differences In Site Engagement Strategies
In rare diseases, training and ongoing communication with study sites are essential. In fact, training is sometimes an even greater focus given there may sites with less research experience and less well-established frameworks in place. In addition, across the Alexion rare diseases, it takes an average of 4.8 years for a patient to receive an accurate diagnosis. Given this challenge, patient referrals may not happen consistently, therefore increasing the importance of communication and study awareness across research sites and with surrounding specialists.
Similarities In Patient Recruitment And Engagement Strategies
The common high-level strategies across both chronic conditions and rare disease include patient insights, education and awareness materials, digital outreach, and travel and support services — even if the execution of them might be different.
Patient insight involves listening to our patients as we are developing medicines for them. AZ has a Patient Partnership Program (PPP) for each of its key indications that allows us to discuss a proposed study’s procedures, visit schedule, and challenges/opportunities. Most recently, one of AZ’s project teams engaged two of our PPP members as part of the core protocol development team. The PPP members participated in protocol reviews and meetings to ensure the team listened and acted upon their feedback. It was an example of a great two-way dialogue and of including the patient voice from the beginning of program development.
At Alexion, a Patient Friction Coefficient (PFC) framework helps us inform and design patient-centric clinical trials by systematically gathering and applying patient insights. Alexion uses the PFC to evaluate the patient and caregiver burden of participation in a rare disease clinical trial in a way that can be measured and replicated. The insights gained help to optimize clinical protocols, develop mitigation plans, and enhance both communication and support services.
As an example, Alexion leveraged its PFC process for a myasthenia gravis Phase 2 clinical trial. Based on the insights gleaned from patients, caregivers, and patient advocacy group (PAG) leaders, Alexion incorporated critical changes to the study protocol, including the addition of rest breaks for visits over four hours, allowance of prior concomitant medication use, as well as patient stipends to cover expenses such as childcare. Moreover, Alexion initiated the development of several tools to help educate patients, including resources to help navigate the trial sites, understand study expectations, and provide appointment reminders through a one-stop-shop app. To address patient self-administration hesitation, we developed an instructional video to give participants the confidence that they could properly administer the investigative treatment. Finally, the learning helped develop a more robust patient recruitment plan, with a focus on patients’ most trusted sources of information: PAGs and healthcare providers.
Educational materials, written in easy-to-understand language, support participants and clearly explain the expectations and requirements of the study. A “toolbox” of materials has been developed so teams are consistent with providing patients with materials, such as general clinical trial awareness material, disease awareness material, and dosing instructions.
In this digital age, AZ is looking to quickly learn what approach best meets the needs of our patients. We have developed an indication level website where if the patient is not currently eligible to join an active study, they are offered the opportunity to join an engagement room where they will stay until they may be eligible, or a site opens in their area. At that point, they will be contacted to let them know their available options and subsequently kept engaged until they identify an appropriate study for them.
Differences In Patient Recruitment And Engagement Strategies
AZ knows through patient feedback that our approach to clinical trial awareness and the specific messaging should be in context to a patient’s disease. For chronic conditions, AZ develops a broad selection of materials (paper and digital) to ensure they appeal to the large variety of needs across patients, the disease, and the globe. We continue to involve AZ’s PPP members in the review of these materials to ensure they answer the patients’ questions and offer further support.
In rare diseases, like with chronic conditions, clinical trial websites and other digital initiatives can provide information that is supportive to patients and their families to learn more. Depending on the rare disease and the associated symptoms, a clinical trial website may serve more as informational and supportive as opposed to being a source of patient enrollment.
As referenced above, travel reimbursement and support services are important components and can make the difference in a patient’s decision to enroll in a clinical trial. For rare disease, travel and related services become more critical, given that patients may reside an even greater distance from the investigation sites. We continue to learn from patients and caregivers through the Alexion PFC sessions described above that sometimes it may not be enough just to provide travel reimbursement. Patients have shared that due to socioeconomic factors, they may not be able to await reimbursement for travel expenses. In another session, a patient reported that she participated in a clinical study and, while grateful for the allowance of a care partner, they were not seated together on the flight and were not able to book adjoining hotel rooms, which was needed at that point for the patient’s care and safety. This example underscores the need to meaningfully understand the patient’s disease and their needs and to have strong communication and relationships with travel providers to set clear parameters to meet those needs.
Processes and frameworks such as AZ’s PPP and Alexion’s PFC help to enable a more patient-centric approach to the design of our clinical trial programs. The insights gained can drive the strategies and tactics needed to support patient recruitment and engagement. We all need to continue to learn from each clinical trial patient and investigative, including the patient in the drug development process and the site through early engagement and feasibility processes to ensure we continue to develop our tools and tactics to support delivery of new medicines.
References:
- About - Genetic and Rare Diseases Information Center. (n.d.). https://rarediseases.info.nih.gov/about
- "Rare diseases: what are we talking about?". Rare diseases centre – Venetian Region – Italy. Retrieved 21 January 2022
About The Experts:
 Deborah Howe is director, global patient recruitment and engagement at Alexion, Astra Zeneca Rare Disease. In this role, she leads the strategic development and implementation of a framework to support planning and delivery of global patient finding and engagement strategies for Alexion Rare Disease studies that leverage patient voice, strategic partnerships, healthcare data, and technologies.
Deborah Howe is director, global patient recruitment and engagement at Alexion, Astra Zeneca Rare Disease. In this role, she leads the strategic development and implementation of a framework to support planning and delivery of global patient finding and engagement strategies for Alexion Rare Disease studies that leverage patient voice, strategic partnerships, healthcare data, and technologies.
She also partners with patient experience and patient advocacy teams to develop and implement friction coefficient activities (patient, caregiver, and investigative site) to assess clinical trial burden with the goal of influencing protocol design and ensuring mitigation and support services are embedded at the study level and based on the insights gained.
Prior to joining Alexion, Deborah worked at Celgene and Bristol Myers Squibb and has over 20 years of experience in patient recruitment in roles of increasing responsibilities and across various stages of drug development and therapeutic areas.
 Michele Teufel is senior director, TA strategy & portfolio delivery within R&I at AstraZeneca. With over 20 years in various roles within clinical operations, Michele has delivered early and late phase clinical studies across different therapeutic areas. In those roles, she ensured project standards and scientific requirements from study design concept through study closeout.
Michele Teufel is senior director, TA strategy & portfolio delivery within R&I at AstraZeneca. With over 20 years in various roles within clinical operations, Michele has delivered early and late phase clinical studies across different therapeutic areas. In those roles, she ensured project standards and scientific requirements from study design concept through study closeout.
In her current role, she leads a team within site management and monitoring (SMM) that supports the respiratory and immunology portfolios. Her team supports collaboration across SMM, therapeutic area, and study management to ensure delivery of the portfolio. Within her team are members of the feasibility team to ensure all projects are planned and set up for success at an early stage.
Acknowledgements
Deborah and Michele would like to thank Liz Bristow, director, patient recruitment and retention, at AstraZeneca for her contribution to this article.
