Introduction To Clinical Trial Site Selection And Activation
Clinical trial sites are the central nervous system of a drug or medical device study, connecting sponsors, investigators, and participants. Sites implement the study’s protocol by recruiting and screening participants, obtaining informed consent, administering the IP or device, providing medical care and monitoring, collecting and managing clinical data, maintaining study documents, reporting adverse events, and communicating with review or ethics boards. Given the scope of these responsibilities, selecting sites is a critical step in study planning that directly influences the trial’s success. This guide covers:
- Clinical Trial Site Selection
- Clinical Trial Site Activation
- Best Practices For Clinical Trial Site Selection And Activation
- Tools And Technologies For Site Selection And Activation
- Challenges And Solutions In Site Selection And Activation
- Emerging Trends In Site Selection And Activation
- Frequently Asked Questions (FAQs)
Clinical Trial Site Selection
Site selection used to depend heavily on networking and manual processes, but targeted strategies and data-driven decision-making are expanding clinical trials to new locations and reaching more diverse populations. However, the human element of site selection remains essential. To participants, sites represent the trial itself, so in-person visits and interviews are necessary to make informed, measured decisions.
Steps In Site Selection
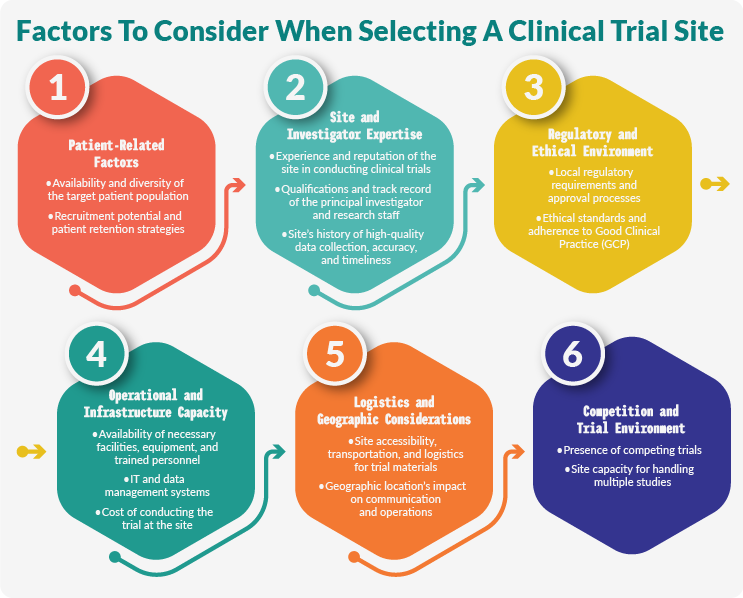
The site selection process entails developing criteria based on the protocol, identifying potential sites, and conducting feasibility studies.
Developing Protocol-Specific Site Requirements
First, researchers use the study protocol to define key site characteristics. This step establishes the desired geographical location, staffing and facility requirements, study duration, and startup timelines. Additionally, researchers determine the participant population and enrollment goals. Researchers must prioritize criteria to ensure that sites have the necessary capabilities to run the trial without unduly narrowing the list of possible sites.
Identifying Potential Sites
Once the protocol-specific criteria are established, researchers identify potential sites using specialized databases, search tools, and professional networks. That initial list should include a variety of sites, including community hospitals, specialized clinics, and primary care facilities.
Conducting Pre-Selection Surveys And Feasibility Assessments
Potential sites are evaluated through surveys and feasibility assessments. First, sponsors send the primary investigator (PI) an inquiry with a study synopsis and a request for the PI’s CV. If the PI is interested and qualified, the next step is to share the study protocol and provide a detailed survey based on protocol criteria. Meanwhile, sponsors gather and review the site’s enrollment track record, regulatory compliance history, infrastructure, costs, and staff expertise.
These surveys should be unambiguous and produce scorable answers that can be objectively compared and analyzed. Sponsors should consider providing additional support in areas such as good clinical practice (GCP) training or patient recruitment tools for sites that also meet the study criteria.

Site Evaluation And Final Selection
Finally, sponsors evaluate and select the best sites for the trial, then notify the sites and send clinical trial agreements (CTAs) to formalize the partnership.
Leveraging Historical Performance Data
Historical performance data like patient enrollment, retention, and protocol adherence are examined to evaluate the site. Data from the previous five years and information on ongoing or planned studies in the same field are especially relevant. Normalized metrics compare site performance across trials and study designs, while data-visualization tools highlight high-performing investigators and sites.
Site capacity should also be considered. Highly sought-after sites frequently engage in multiple trials, which could stretch their resources and limit their ability to handle new studies efficiently.
Assessing Regulatory Compliance
Sponsors also evaluate a site’s regulatory compliance track record and look for the following red flags:
- Protocol deviations or missed deadlines
- Adverse findings from past audits
- Inadequate documentation of previous trials
- Poor track record in data submission or patient safety concerns
- Lack of robust patient safety protocols and transparent processes for monitoring adverse events
- Insufficient or outdated data management systems
- Inadequate informed consent procedures
- Conflicts of interest or exploitation of vulnerable populations
- Lack of transparency in contractual arrangements with vendors or other trial participants
- Unclear payment structures, including requests to be paid in different countries or through separate legal entities
- Guarantees of expedited regulatory submissions or patient recruitment without disclosing the approach
- Incomplete or inaccurate medical records
- Missing policies or inconsistent coding practices
Considering Geographical Factors
Sponsors also should assess a site’s local healthcare infrastructure and any potential site congestion in areas where multiple trials for the same indication are already taking place. For the latter, one option is to consider working with a qualified but research-naïve site. Country and regional trends, as well as cultural and technological factors, also affect site performance.
Performing On-Site Visits, Investigator Meetings, And Interviews
The last evaluation stage entails direct interaction with potential sites. In-person visits help sponsors evaluate investigators, staff, facilities, and equipment firsthand to ensure that the site is fully capable of managing the trial.
Clinical Trial Site Activation
Once sites are selected, they undergo the activation process to prepare them to begin enrolling participants and running the study. Site activation can be lengthy, so sponsors should aim to standardize and streamline it as much as possible.
Steps In The Site Activation Process
Site activation is a multi-stage process that prepares the site to meet regulatory and ethical standards, prove financial viability, and create operational readiness.
Regulatory And Ethics Committee Approvals
Before the study begins, regulatory authorities and ethics committees rigorously review the protocol. In the U.S., the FDA reviews IND applications for drug products, while institutional review boards (IRBs) evaluate the study’s ethics. Criteria include scientific merit, potential risks and benefits to participants, and informed consent procedures. This process can take several months, but regulatory and ethics committee approvals must be granted before enrollment begins.
Contract And Budget Negotiations
Contract and budget negotiations often run parallel to the regulatory approval process. Sites require funding for personnel, procedures, and overhead expenses, which vary according to the protocol and the site’s cost structure. Sponsors, CROs, and sites work together to negotiate the study’s financial and legal framework, and the resulting CTA details all parties’ responsibilities, intellectual property rights, publication terms, and other trial aspects. Ideally, the relationship is mutually beneficial, with the site receiving adequate support to run the study and sponsors receiving an efficient, timely, and productive trial.
Training And Site Initiation
Lastly, staff training and site initiation prepare site personnel and infrastructure for the study. Typically, this stage begins after the contract is executed and regulatory approval is secured to ensure the site can safely conduct the trial while maintaining data integrity and regulatory compliance.
The study team is trained in the protocol, GCP guidelines, and study-specific procedures. Typically, sponsors or CROs conduct a site initiation visit to ensure the site is fully prepared to start enrolling participants. They review the protocol, discuss recruitment strategies, and verify that all required equipment, supplies, and documentation are in place. In addition, the site’s data management systems and procedures for managing adverse event reporting are evaluated.
Streamlining The Activation Process
Strategic planning can streamline activation, reducing startup timelines. First, initiating the process during the clinical design stage helps sponsors identify potential bottlenecks and implement mitigation measures, improving resource allocation.
Standardizing and simplifying processes also streamlines activation. SOPs should cover contract negotiation, ethics review, and regulatory submission to minimize back-and-forth communication and reduce errors. Likewise, contracts and budgets can be standardized to accelerate negotiations and CTAs.
Technology such as EDC, CTMS, and e-consent also can improve activation timelines, expedite document collection/review processes, and reduce manual errors.
Clear communication channels for sponsors, CROs, and sites are essential to ensure all parties are informed and aligned. Comprehensive site staff training in the study protocol, procedures, and data management minimizes deviations and errors.
Best Practices For Clinical Trial Site Selection And Activation
Best practices improve site selection and activation, creating more efficient clinical trials. Data-driven decision-making, effective patient recruitment strategies, strong sponsor-site relationships, and budget optimization help sponsors overcome common challenges and accelerate study timelines.
Leveraging Data-Driven Decision-Making
Increasingly, sponsors rely on advanced data collection and analysis tools to enhance the site selection process. Historical performance data identify top investigators and sites, while data visualization tools compare sites’ performance across different trial types and study designs. The shift from intuition-based decisions to data-driven insights allows sponsors to accurately predict site performance and reach their target participant population.
Improving Patient Recruitment
Patient recruitment remains a significant hurdle, but social media, search engines, and traditional media outlets can be leveraged to reach a broad audience of potential participants. Community outreach and referral systems also raise awareness, and partnering with specialty clinics focused on particular medical conditions targets interested individuals. Adopting patient-centered approaches also makes the trial more appealing and feasible to potential participants.
Building Strong Sponsor-Site Relationships
For activation to run smoothly, sponsors, investigators, study sites, and vendors must collaborate and communicate frequently. Clear communication channels and transparency among stakeholders help identify potential cost-saving opportunities and improve decision-making.
Site staff also need comprehensive training on the protocol and ongoing support. Performance-based incentives and regular updates keep staff engaged in the trial’s success. Sponsors should also meet with sites and investigators regularly to ensure the trial is following the protocol and meets its primary objectives.
Budget Optimization Strategies
Clinical trials significantly increase drug development’s price tag, so budget optimization is crucial to conserving resources. Streamlined study designs and procedures focusing on primary objectives and endpoints can reduce costs. A risk-based monitoring strategy also optimizes resource allocation and reduces on-site monitoring costs. Additionally, choosing sites with successful track records in participant recruitment, retention, and protocol adherence helps sponsors stay on budget.
Expenses should be regularly tracked and managed throughout the trial, and periodic budget reviews can identify areas for cost savings. Finally, alternative funding sources and partnerships with academic institutions or government agencies can provide additional financial support.
Tools And Technologies For Site Selection And Activation
Advanced tools and technologies are vastly improving the site selection and activation process by enabling efficient, data-driven approaches that streamline processes and provide ongoing performance monitoring.
Digital Tools For Streamlining The Process
Advanced platforms are accelerating site activation. For instance, investigator site portals create a consistent experience across trials, offering sites precise documentation that is customizable by country and region. They also improve the regulatory submissions and approvals process and include reminders and alerts to keep processes on track.
Additionally, digital data collection, site management, and communication tools drastically reduce manual errors, enable real-time stakeholder collaboration, and support remote monitoring. Real-world data (RWD) platforms help researchers cast a wide geographical net to find potential trial participants from diverse populations and identify community-based sites like clinics and hospitals. Further advanced technologies combine data and AI to tap into a network of providers and patients to speed up enrollment and site activation.
Enhancing Performance Monitoring
Modern tools and technologies enhance performance monitoring, leading to data-driven site evaluations and continuous quality improvement.
KPIs For Site Evaluation
Some CTMS will offer suites of KPIs to evaluate site performance and provide real-time insights into patient enrollment rates, data quality, protocol adherence, and site efficiency. Digital dashboards create KPI visualizations, allowing study managers to identify trends and rapidly address issues. KPIs that benchmark performance across different sites and studies include:
- Time to first patient contact
- Patient recruitment and retention rates
- Data entry timeliness and accuracy
- Protocol deviation rates
- Site initiation efficiency
- Time to data entry
- Staff turnover rates
Challenges And Solutions In Site Selection And Activation
The site selection and activation process carries many challenges, including low enrollment, outdated methodologies, and activation delays that lead to wasted resources and slower drug development timelines.
Common Hurdles In Site Selection
Under-enrollment is a common issue with site performance. According to the Tufts Center for the Study of Drug Development, 37% of sites do not meet enrollment quotas, and 11% don’t enroll any participants. Over time, 89% of studies meet their enrollment goals by extending timelines or engaging more sites, but only after costly delays.
Secondly, the site selection process often relies on outdated manual processes that are error-prone and lack transparency. Sites frequently overestimate their enrollment capabilities, highlighting the need for high-tech, data-driven decision-making.
Overcoming Selection And Activation Delays
Activation delays are another common problem but can be overcome using best practices and digital technologies such as:
- Enhancing transparency via digital trial platforms allows sites to view and select trials, fostering engagement and responsiveness.
- Reducing the questionnaire burden by focusing on essential questions accelerates site evaluations during the selection phase.
- Just-in-time site activation engages sites in the activation process before protocol approval. Master CTAs, budgetary items, and essential site documents are all prepared and collected in advance to prepare for activation.
- Prioritizing site activation means that sponsors strategize activation while waiting for approval to launch the study quickly.
- Early staff training and cross-trial training for site staff speed up activation.
- Streamlining processes like implementing electronic confidentiality statements reduces time-consuming manual labor.
Emerging Trends In Site Selection And Activation
Site activation trends aim to shorten timelines, improve efficiency, and create patient-centric trials. For example, digital trial platforms enable better communication and collaboration between sponsors, CROs, and sites throughout the activation process. Sponsors also turn to study startup specialists who work directly with sites during activation, providing expertise in specific regulatory guidelines and serving as a single point of contact.
RWD is transforming site selection by identifying existing patients and their closest treatment sites. This method casts a wide enrollment net, identifies community-based sites that may be overlooked, and improves diversity and inclusion. Furthermore, incorporating decentralized elements like telemedicine may expand enrollment reach into rural areas far from hospitals or clinics.
AI and ML can also be used on historical data to enhance data quality and analysis to better help sponsors make informed, objective decisions regarding site selection.
Conclusion
Selecting and activating high-performing sites is crucial to launching efficient, cost-effective clinical trials that meet their primary objectives. Enrollment and activation challenges frequently slow clinical trials and add to their already high costs, but new technologies and the use of objective data points promise to improve these processes and speed up drug development timelines.
Frequently Asked Questions (FAQs)
Below are frequently asked questions regarding site selection and activation.
1. What are the key factors when selecting a clinical trial site?
Key factors include geographical location, local population demographics, site staff expertise, facility requirements, study duration, and target timelines. The site’s track record in patient recruitment and retention is also crucial.
2. How can sponsors streamline the site activation process?
Sponsors can streamline site activation by initiating the process early in the trial design phase, standardizing processes, utilizing electronic document management systems, simplifying contracts and budgets, and leveraging technology for training and communication.
3. What are some emerging trends in site selection?
Emerging trends include using RWD to identify potential trial participants, leveraging AI and ML for data-driven site selection, and adopting a patient-centric approach to reach diverse populations.
4. What is "just-in-time" site activation?
Just-in-time site activation involves engaging sites in the activation process before protocol approval, executing master CTAs, and pre-negotiating certain budgetary items to accelerate the activation timeline.
5. How are digital tools improving site selection and activation?
Digital tools enhance the process by providing real-time insights into site performance, offering comprehensive KPI tracking, enabling remote monitoring, and facilitating better communication between sponsors, CROs, and sites throughout the activation process.
6. What are some common challenges in site selection?
Common challenges include poor site performance leading to under-enrollment, manual and error-prone selection processes, sites’ inability to accurately predict enrollment rates, and regulatory complexities.
7. How can sponsors ensure diversity and inclusivity in site selection?
Sponsors can ensure diversity by leveraging RWD, considering areas where target populations live, addressing potential travel barriers, and evaluating sites’ ability to engage with diverse patient populations.
About The Author
Elizabeth Mann is a skilled writer with over a decade of experience in content creation, specializing in the life sciences industry. As a writer for Life Science Connect, she develops in-depth content that informs and engages professionals in the pharmaceutical, biotechnology, and medical device sectors. Her areas of focus include biologic drug production (including cell and gene therapies), clinical trial design and execution, and drug development and manufacturing outsourcing.
EXPERT INSIGHTS ON SITE SELECTION
-
4 Crucial Differences Between Early-Phase And Late-Phase Trial Site Selection
Learn the differences between early-phase and late-phase trial site selection, including what makes an ideal site for early-phase trials, where speed, precision, and adaptability are non-negotiable.
-
From Data Chaos To Clarity: How To Modernize Clinical Trial Site Selection
The Pistoia Alliance introduces the ClinOps Ontology Project, an effort to establish a common language agreed upon by stakeholders across the industry to improve and standardize data collection and streamline site selection.
-
Site Selection Directly Impacts Diverse Patient Enrollment In Rare Disease
Adaptive Research Inc.'s Deepak Behera, MD and Melissa Andrews make the case for community trial sites as the go-to source for ensuring more diverse patient populations in rare disease clinical trials.
-
Best Practices For Trial Design And Site Selection In Pediatric Clinical Trials
“Children are not small adults," reminds Katie Smentek, MD, a PI at Mankato Clinic. Here, Smentek shares her advice for designing trials and selecting sites for pediatric patients.
-
No CRO? No Problem As Curadev Tackles Site Selection For Its First Clinical Trial
Curadev COO and CFO Manish Tandon discusses the company's first foray into site selection (all without a CRO), covering a site’s influence on patient recruitment and centricity.
EDITORIAL PERSPECTIVES ON SITE SELECTION
-
Being Financially Prudent As A Site Isn't a Bad Thing
Most sites are composed of three silos of people who rarely cross communicate -- especially about issues that could affect finances. But if your job is to take care of patients. should you be concerned about the same things as your finance-related colleagues? Dr. Daniel Fox says, at the very least, you should be communicating some key events/milestones.
-
Should We Be Scared Of PE Firms Investing In Sites?
It seems lately there's been a lot of vilification of private equity (PE) firms that are purchasing site networks. I'm sure this sentiment has increased during the past few years, but is it justified?
-
Site Networks Find Strength In Numbers With AMRC
Jim Kremidas, executive director of the newly launched Association of Multisite Research Corporations (AMRC), talks about the genesis and future of this association, which launched with 14 multisite clinical research corporations (MCRCs) members.
-
For Hybrid Trials, Have Some Faith … In Sites
Noelle Gaskill believes the key to regulatory success with a DCT is to not force a bunch of tech onto sites, but instead, choose those that already have the needed technology and experience and then let them do what they do best – execute on the trial.
-
Why Can't We Compare Site Performance Measures?
Cerdi Beltré believes it’s possible to develop a global and objective way to measure investigator site performance for metrics such as startup duration, screen-pass rates, or rate of enrollment. It just won’t be easy … or make some sites happy.
ON-DEMAND SITE SELECTION WEBINARS
-
Elevating The Site-Sponsor Experience: SaaS-Based Feasibility And Site Selection For Clinical Trials
In this webinar, learn about the transformation clinical trial feasibility and site selection across the industry.
-
Operational Innovations That Enhance Site-Sponsor Collaboration
Overcome obstacles associated with collaboration that, when left unaddressed, lead to site selection and patient enrollment issues for sponsors and operational challenges for sites.
-
Leveraging AI And Machine Learning To Deliver Next Generation Feasibility & Site Selection
In this presentation delivered at SCOPE 2022, hear how ICON leveraged Study Feasibility to its advantage.
-
Leveraging Internal And External Insights For Precision Enrollment Modeling
Discover a trial feasibility tool that integrates users' operational insights with high-quality clinical intelligence to drive accurate and informed enrollment projections and site capacity analyses.
-
Addressing The Challenges And Opportunities In Gynecologic Cancer Research
With investment in research on the rise, explore the challenges investigators face in patient identification, site selection and the ever-changing regulatory landscape in the available webinar.