Realizing the Promise of Data Sharing: A Multi-Stakeholder Collaboration
By Aaron Mann, senior vice president, Clinical Research Data Sharing Alliance

Sharing patient data generated from clinical trials is fundamental to the advancement of science and improvement of public health. The use cases for shared data are well-established and include novel clinical trial design and enrichment strategies, predictive preclinical and clinical models, clinical trial simulation tools, biomarkers, clinical outcomes assessments, and more.1 Shared clinical research data also has the power to transform the trial process itself, improving the patient experience and delivering life-saving and life-changing therapies faster and at less cost to society.
Clinical trial data is collected with the patient’s consent, from trials designed with clearly defined endpoints and objective assessments. It represents some of the highest-quality research data available. Yet data sharing platforms are challenged to get enough trials contributed, in time frames that meet researcher needs, and with the consistent data utility needed to enable meaningful analysis.
Without effective reuse of both clinical trial and academic research study data, it’s clear that no organization, no matter how large, has enough, or the right, data across all therapeutic areas they work in. Further, we have an ethical responsibility to the patients, who donate their time and their data in the trial process, to use their data to the fullest. However, today’s logistical and policy challenges mean that this regulatory-grade data-at-scale is often an underutilized driver of innovation.
The Clinical Research Data Sharing Alliance (CRDSA)
Realizing the promise of data sharing requires addressing many challenges across all stakeholder groups. Significant advances have been made, but because efforts are often targeted at a specific use case or stakeholder group, resulting solutions may be fragmented or incomplete when viewed with an ecosystem-wide lens. This creates significant data use barriers for researchers and ultimately slows the advances needed to develop life-saving and live-improving therapies, diagnostics, and devices.
In early 2021 a group of like-minded data sharing experts from across the stakeholder landscape recognized that the challenges couldn’t be solved in isolation, by a single platform or by a single data contributor. It was clear we needed to work together to make it easier for data contributors to share and for researchers to use those data.
The Clinical Research Data Sharing Alliance (CRDSA) is a new multi-stakeholder consortium that serves as an umbrella organization for the clinical data-sharing ecosystem. Our members include biopharma companies, nonprofit data sharing platforms, academic institutions, and service and technology partners. This diverse and growing group of stakeholders comes together with the shared goal of accelerating the discovery and delivery of life-saving and life-changing therapies to patients by expanding the research value of the high-quality data collected through the clinical trial process.
CRDSA is not a data sharing platform, nor is it a data contributor. Rather, it serves as a collaborative forum supporting stakeholders across the data sharing landscape:
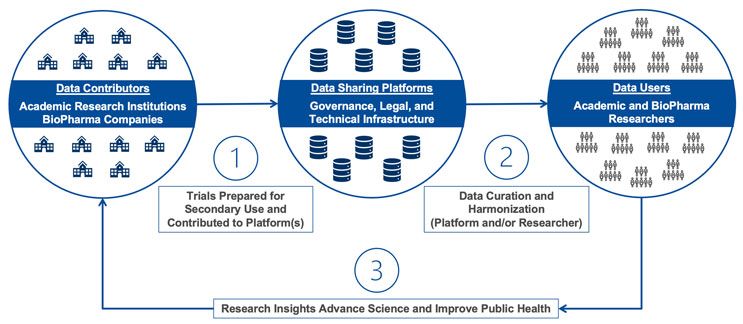
Figure 1: Data Sharing Landscape
CRDSA supports this ecosystem in three ways:
- First, we provide a collaborative forum for all stakeholders to come together to address logistical obstacles to the effective contribution and use of shared trial data.
- Second, we ensure the resulting solutions are consensus-based, have broad multi-stakeholder support, and can serve as an accepted best practice reference point for data contributors and researchers.
- Third, we recognize technical and process solutions often require advancements in regulatory and organizational policies to be fully realized. CRDSA creates the unified multi-stakeholder voice needed to effectively advocate for meaningful policy change.
By working together, we can create solutions that reduce the logistical data-sharing hurdles and maximize the value of patient-donated data. But if the benefits of using these data are clear, why is data sharing so hard? And what can we do about it?
Why Is Data Sharing So Difficult?
Sponsors are starting to heed the calls from groups like the World Health Organization,2 the National Institutes of Health,3 the G7,4 and patient groups to widely share patient-donated data. However, calls for open science must be balanced against an equally important need to protect patient privacy. Exacerbating the situation is the changing landscape of national and international regulatory requirements.5
These two sides of the spectrum essentially create a governance continuum. Where an organization falls on that continuum determines a range of success factors, including adequate resource allocation, a balanced data protection approach, and proactive data preparation.
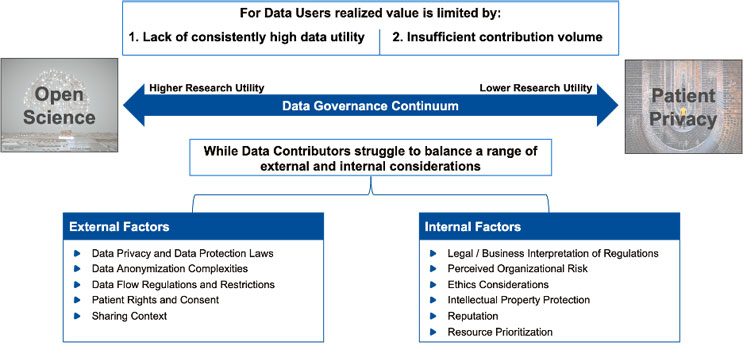
Figure 2: Data Sharing Governance Continuum
Beyond policy considerations, it’s also important to recognize there are practical challenges organizations face in contributing data. Both biopharma companies and academic research institutions may lack clear-cut processes for determining which studies can be shared externally. Even under a defined data governance policy, the decision to share a specific trial must take into account factors including IP protection and the ability to protect patient privacy. Once the decision to share is made, there is then a very real resource cost in preparing data for contribution.
Against this background, data contributors often struggle to share trial data and when data is shared, secondary use data transformations (e.g., anonymization) may compromise data utility to the point where meaningful analysis becomes difficult or even impossible. It’s then left to each data sharing platform to try to resolve these issues, exacerbated by a lack of FAIR (findable, accessible, interoperable, and reusable) data standards that facilitate interoperable reuse between platforms.
CRDSA’s Work Streams: Pragmatic Delivery
CRDSA seeks to remove impediments to effective data sharing. To do so requires developing shared solutions and making changes to regulatory policies to ensure consistent acceptance of secondary data in the clinical development life cycle. CRDSA’s workstreams address specific challenges that have been identified as priorities and are designed to support long-term objectives with actionable and achievable deliverables.
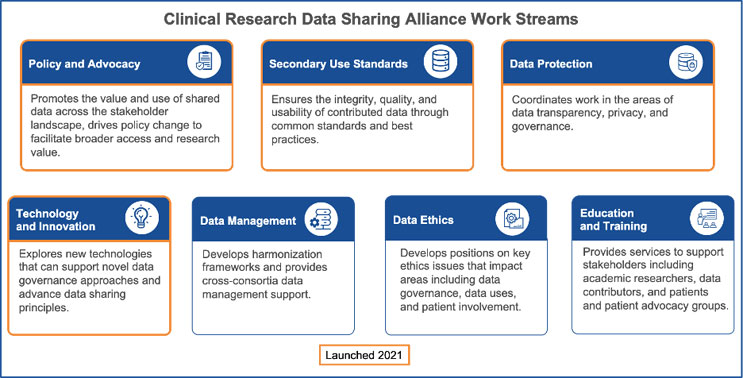
Figure 3: CRDSA Work Streams
CRDSA launched four workstreams in Q4 2021:
1. Policy and Advocacy: Organizational policy determines everything from resourcing to data privacy approaches, while regulatory policy is the framework in which data sharing governance operates. Our work in this area has started with two projects:
Regulatory Acceptance of Supplemental Controls: Regulatory acceptance in confirmatory trials is perhaps the most important potential benefit of data sharing because it can reduce the patient burden and the time and cost for patient recruitment. Multiple consortia and sponsors are already working with health authorities on policy advancement; however, these efforts are largely siloed. To support these efforts, CRDSA is creating a forum to identify synergies, common challenges, and opportunities for collaboration.
Data Contributor Strategic Messaging: Support from executive leadership within organizations is foundational to facilitating successful external data sharing. The project deliverables include a customizable strategic messaging toolkit designed for senior audiences.
2. Secondary Use Standards: Advancing FAIR data principles is key to supporting data sharing platforms and the researchers they serve. The first project in this workstream is developing a common metadata specification that gives end users the information needed, including any data transformations applied, to determine the overall suitability of a specific study for their research needs. The intent is to reduce the burden on both contributor and user by ensuring key information is communicated before data management activities.
3. Innovation and Technology: Next-generation technologies may enable new approaches to privacy protection, data governance, and analysis. This workstream is charged with assessing emerging technology models to evaluate their relative maturity, applicability, and best-fit use cases.
4. Data Protection: A significant body of work has been done and continues to be developed in the areas of data transparency, privacy, and governance. Guidance, definitions, and approaches may conflict, causing confusion within and across organizations. To avoid duplicative or competing efforts, CRDSA will work with the appropriate organizations and stakeholders to coordinate existing and planned efforts. A forward-looking gap analysis will be used to inform future CRDSA initiatives and coordination activities. By representing a unified data-sharing “voice,” CRDSA is well-positioned to advocate for advancements in privacy policies.
Conclusion
Broad access to these data has the power to transform the research process, improve trial design and delivery, and benefit the patients who donate their time and their data as part of the clinical development process.
Together, we can drive the value and use of clinical research data to more efficiently deliver life-saving and life-changing therapies to the patients we serve. CRDSA welcomes organizations from across the data sharing ecosystem to join us in transforming the future of data sharing. To find out more about CRDSA, and how your organization can get involved, please visit https://crdsalliance.org.
“When communities come together with a shared goal to collaborate, the profound impacts of patient-level data sharing are realized by individual organizations and, most importantly, patients. Reaffirming and expanding our commitments to effectively sharing data for the betterment of public health will result in dramatically improved medical product development and more rapid development of life-changing therapies for patients.”1
References:
- Karpen, S.R., White, J.K., Mullin, A.P. et al. Effective Data Sharing as a Conduit for Advancing Medical Product Development. Ther Innov Regul Sci 55, 591–600 (2021). https://doi.org/10.1007/s43441-020-00255-8: 591, 599
- Joint Statement on Transparency and Data Integrity; International Coalition of Medicines Regulatory Authorities and WHO; May 7, 2021: https://www.who.int/news/item/07-05-2021-joint-statement-on-transparency-and-data-integrityinternational-coalition-of-medicines-regulatory-authorities-(icmra)-and-who
- Dr. Francis S. Collins; National Institutes of Health Statement on Final NIH Policy for Data Management and Sharing; October 29, 2020: https://www.nih.gov/about-nih/who-we-are/nih-director/statements/statement-final-nih-policy-data-management-sharing
- G7 Research Compact; Cornwall UK 2021: https://www.g7uk.org/wp-content/uploads/2021/06/G7-2021-Research-Compact-PDF-356KB-2-pages-1.pdf
- Volchenboum, S., Plana, A., Fumer, B. et al. Pediatric Cancer Data Commons: Federating and Democratizing Data for Childhood Clinical Research. DOI: 10.1200/CCI.21.00075 JCO Clinical Cancer Informatics no. 5 (2021) 1034-1043. Published online October 18, 2021: 1038
About The Author:
 Aaron Mann is senior vice president, data science, at the Clinical Research Data Sharing Alliance (CRDSA). Recognizing the data sharing opportunities and challenges across the landscape, he led the multi-stakeholder effort behind the establishment of CRDSA in 2021. Prior to joining CRDSA, he was Roche and Genentech’s global program lead for Industry Collaborations Data Sharing Initiatives and served as the industry lead for DataCelerate, TransCelerate BioPharma’s data-sharing platform. His experience spans global enterprises, high-growth technology companies, and early-stage startups, with a focus on developing data governance and data analytics strategies for companies including Visa, SanDisk, Symantec, and Capital One. You can contact him at: aaron.mann@CRDSAlliance.org or https://www.linkedin.com/in/aaronmann/.
Aaron Mann is senior vice president, data science, at the Clinical Research Data Sharing Alliance (CRDSA). Recognizing the data sharing opportunities and challenges across the landscape, he led the multi-stakeholder effort behind the establishment of CRDSA in 2021. Prior to joining CRDSA, he was Roche and Genentech’s global program lead for Industry Collaborations Data Sharing Initiatives and served as the industry lead for DataCelerate, TransCelerate BioPharma’s data-sharing platform. His experience spans global enterprises, high-growth technology companies, and early-stage startups, with a focus on developing data governance and data analytics strategies for companies including Visa, SanDisk, Symantec, and Capital One. You can contact him at: aaron.mann@CRDSAlliance.org or https://www.linkedin.com/in/aaronmann/.
