Takeda's Diversity Strategy Pays Off For Phase 3 Trial

By Dan Schell, Chief Editor, Clinical Leader

In a broad sense, DEI initiatives, projects, and teams are being diminished, shelved, or outright dismantled. But thankfully, that’s not the case when we look at diversity in clinical trials. Irrefutable science and data — as well as some new regulations — dictate that we need to maintain our momentum.
In the past two years, thanks to two members of my editorial board, Denise Bronner and Karen Correa, I’ve learned a lot about diversity in clinical trials focused on dermatological diseases. So I was intrigued when I heard that Takeda had met or exceeded nearly all of its enrollment goals for underrepresented populations in its Phase 3 trial of zasocitinib, which targets patients living with psoriasis. To get more info, I spoke with LaShell Robinson, head of global feasibility and trial equity at Takeda.
Hitting That Enrollment Goal 6 Months Early
Before we go any further, here are three key bullet points that will set the stage for you on this topic:
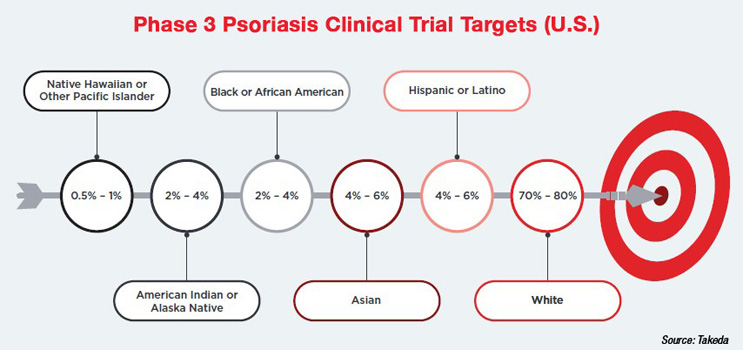
- Psoriasis is a chronic inflammatory disease that affects over 7.5 million Americans and 60 million people worldwide.
- Clinical trials for psoriasis have historically been the least diverse within dermatology, predominantly featuring white males, which limits the understanding of treatment responses across different patient populations.
- Zasocitinib offers the potential for an oral treatment option, which could be a significant advantage for patients compared to injectable biologics.
Although specific metrics cannot yet be shared due to ongoing regulatory submissions, Robinson notes that Takeda more than doubled its enrollment goal for Hispanics and nearly doubled its goal for Black/African Americans. The trial did fall short of its goal for the Native American population, but its enrollment still exceeded representation in the U.S. population. Finally, and perhaps most importantly, enrollment ended six months ahead of its goal.
Obviously, you don’t achieve those types of positive stats without some serious planning.
For Robinson and her team, that planning starts at the protocol synopsis phase. They use data from multiple sources, including literature reviews, real-world evidence, site-reported data, and claims data, to establish more accurate enrollment goals and to help choose the countries where the trial will be run. “After that, the operational components begin,” she says. “For instance, we send out site feasibility questionnaires, which include a question about a site’s reported demographics. We also ask about their level of community engagement.”
What Exactly Is “Boots On The Ground?”
Community engagement is part of Takeda’s PAVE framework, which they’ve been integrating into all their trials since June 2022, regardless of the phase. It stands for:
- Partnering with community stakeholders
- Addressing operational barriers
- Verifying goals based on real-world data
- Enhancing the diversity of investigative sites
A lot of companies talk about their commitment to the community and getting involved, so I wanted to know details of what Takeda is doing in this regard. First, Robinson explained that no matter what the environment or the topic of the trial, much of the education they provide at community events is simply about clinical research in general. In fact, she has even gone on local radio shows where people can call in and ask her about clinical research.
She told me the story of one PI who had been given the approved educational materials about plaque psoriasis, but felt it was more important to explain the basics of clinical research to the community rather than the details of the trial itself. “His point was that he couldn’t get them talking or even considering a trial if they didn’t know the basics of clinical research, like what a placebo is. After that, he could talk with them about the trial itself and explain why it's so important that there's representation from their community and why this can help advance science.” So, on the hottest day in New York a couple of years ago, he showed up at a church that was primarily Latino to give his presentation. Takeda also sent patient recruitment and clinical operations staff. The PI then asked if he could give the presentation in Spanish, and of course the answer was yes.
Beyond the typical health fairs, Takeda also has found success engaging with other community groups like fraternities and sororities. For example, they’ve connected with Alpha Phi Alpha Fraternity, the oldest intercollegiate historically African American fraternity. “We’ve met independently with various local chapters of this fraternity, but for one we partnered with one of our trial sites, which was really exciting for our plaque psoriasis trial, in particular,” Robinson explains. “We are trying to encourage sites to be part of our community engagement efforts, which isn’t something we’ve traditionally asked them about, and for many, is a new experience. But now, it is part of that early planning I previously mentioned when we’re focused on diversity in trials.”
Bridging The Education Gap In Dermatology Trials
Robinson reiterated something I had learned from my conversations with Correa and Bronner, namely, that images of skin of color are often missing from medical textbooks, which creates a gap in diagnosis and understanding. To bridge this gap, Takeda incorporated diverse images into their protocol slides and conducted a training session at the investigator meeting led by a key opinion leader specializing in diagnosing skin of color. This session covered the nuances of diagnosing psoriasis on different skin tones, such as the fact that it may appear gray on African-American skin.
Furthermore, Takeda created a "patient experience workshop" to explore cultural nuances and address potential barriers to clinical trial participation. By encouraging sites to consider the unique experiences and questions of diverse patients, Takeda aimed to create a more inclusive and welcoming environment.
Advocacy Group Partnerships
Despite the successes surrounding Takeda’s Phase 3 psoriasis trial, Robinson acknowledges that there is much more work to be done. For example, they’d like to have enrolled more females for this trial, even though they didn’t establish enrollment goals for gender.
Takeda also recognizes the importance of partnerships with patient advocacy groups to amplify its diversity efforts. “For this trial, we did a podcast with the National Psoriasis Foundation where we explained our PAVE program step by step,” Robinson says. “We wanted to assure patients that we're really taking responsibility for setting this up for success. We are also meeting with other advocacy groups [she cannot reveal who at this time] globally to discuss best practices both of us have realized. One of those topics is the imagery being used for dermatological diseases. We are making sure we're elevating that one because it’s a huge issue.”
Editor’s note: Takeda expects to share the specifics of this trial’s data by the end of 2025.
