
DECENTRALIZED TRIALS

The Impact Of AI On Mobile Visits
Experts discuss the FDA's new AI guidance, global harmonization, and the ethical implications of AI in clinical trials. Discover the challenges and opportunities ahead.

Patient-Centric Cardiac Monitoring For Decentralized And Hybrid Trials
A patient-centric approach to cardiac monitoring is helping to advance data collection and reliability during decentralized and hybrid clinical trials.

Everything You Need To Know About Adopting eConsent Across Large Pharma Organizations
Did you know that the average cost for a delayed trial is between $600,000 and $8 million per day? Discover how electronic consent management technology is helping sponsors, CROs, and sites.

What It Really Takes To Adopt eConsent Across Large Pharma
Explore the benefits of incorporating consent management technology and discover practical strategies for developing effective change management and training programs.
The New Vaccine Race: Why A Decentralized Approach Wins
What does it take to get vaccine trials up and running quickly and efficiently? As it turns out, the best answer is DCT. Learn why in the available webinar.

Trials And Tribulations Of Electronic Patient Consent: Removing Barriers To eConsent Adoption
After a decade of dabbling with eConsent, how can sponsors and technology providers meet the demand to bring clinical trials directly to the patient at a global scale? In this webinar, you’ll learn strategies to overcome the persistent challenges in eConsent adoptio...

DCTs: The Site Perspective
Putting patients and sites on the same platform coordinates and simplifies eCOA patient data collection and provides a 360 degree view of the patient. See how through Dr. Aaliyah's story.
Medidata Decentralized Clinical Trials Program
Watch this brief video to see how the industry’s only scalable, end-to-end offering for decentralized clinical trials can connect trial experiences for patients, sites and sponsors.

Elevating The Site Voice
A decentralized trial can be daunting for sites, but Medidata offers solutions to help site staff, sponsors, and patients move smoothly through the clinical trial process.

The New Era Of Evidence Generation In Clinical Trials
Learn how top pharmaceutical companies are using novel innovations to drive greater speed, scale, and access in clinical research than ever before in this webinar hosted by Musaddiq Khan, Vice President of DCT Solutions at Medable.
Transformative Cardiac Solutions
While traditional ECGs require on-site visits, delaying data and increasing drop-out risk, AliveCor''s FDA-Cleared ECG devices can be used on-site as well as in decentralized and fully remote trials.

Collect Better Oncology Trial Data, Easier
In this webinar, experts discuss how enriching oncology clinical trials with RWD generates deeper insights and improves operational efficiency to achieve better patient outcomes.

The Evolving Role Of AI And Innovation In Cardiac Monitoring
In this presentation, the panel of experts focuses on the changes AI has brought to cardiac monitoring data during clinical trials and addresses the skepticism over the use of new innovations.

Enable Next-Generation Clinical Research At Scale
In this webinar, Medable SMEs take you through the end-to-end process of conducting decentralized clinical research, including benefits, common pitfalls and challenges, and more.

Digital Physical Activity Measures In Phase 3 Immunology Trials
Dr. Jie Shen, Research Fellow and Director of Digital Science at AbbVie, shares lessons learned from incorporating digital measures of physical activity into Phase 3 immunology clinical trials.

Medable Total Consent: The Future Of Informed Consent
With Total Consent, Medable is transforming the consenting process – harmonizing it into one simple-to-use platform that enables trial sponsors and CROs to completely customize eConsent.

How State-Of-The-Art Clinical Trial Mobile Vision Clinics Work
Are you seeking a non-traditional option to complete the eye exams necessary for your ocular clinical trials? A mobile clinic may be the solution you're looking for.

Harness The Power Of eCOA In Your Next Trial
In this webinar, explore the importance of eCOAs, how they have evolved from the standard paper diaries, and what efficiencies you can realize from them.

Three Data Trends to Consider Now When Developing Your Decentralized Clinical Trial Strategy
In this free webinar, learn how to update your clinical data strategy that ensures you can scale trials globally while decreasing the overall burden on sites and patients.
Leveraging DHTs To Improve Data Collection And Analysis
How can wearable DHTs be leveraged to measure physical activity in patients with immunological diseases, enhancing clinical trials and advancing drug development?
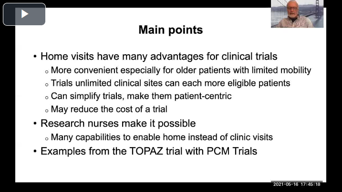
Clinical Trials From Home: The Role Of Research Nurses
In this presentation, speakers shed light on the advantages of home visits for clinical trials and what research nurses do to make this possible.

Transforming Cardiac Monitoring With 6-Lead And 12-Lead Devices
Learn how you can monitor a large number of cardiac conditions in real-time and support clinical trials across phases and TAs.

Creating The Digital Foundation For Scale In Clinical Development
Learn how CROs, pharma, and biotechs are using digital trial methods to scale efficiencies across the entirety of their clinical trial portfolio for maximum ROI.

Modernizing Clinical Research With Patient-Centric Digital Data Collection
Accelerate clinical trial modernization with streamlined collection of continuous, high-precision data centered on the patient.

Patient First Solutions For Oncology
Medable, Inc's new oncology offering is an end-to-end suite that includes an extensive eCOA library, pre-built and validated DCT applications such as Total Consent and Televisit, and protocol design consulting.
The Past, Present, And Future Of Mobile Visits In DCTs
From pandemic-driven adoption to a new era of patient-centric research, experts share insights and strategies for the future of decentralized clinical trials (DCTs).

Site Support: The Cornerstone Of The Digital Trial Evolution
Follow along as this panel of experts shares key insights from their experiences, highlighting the technologies and processes that lead to successful partnerships in patient-centric digital trials.

Applying The Lessons Of The Pandemic To Your Upcoming Trial
In this webinar featuring Ellen Weiss, vice president of in-home solutions for PCM Trials, learn how the pandemic has impacted the industry and what it means for CROs and sponsors now and moving forward.

A Framework For Selecting Reliable Clinical Endpoints From Real-World DHT Data
Dr. Andy Liu presents a case study determining which digital measure of physical activity would be a well-defined and reliable clinical endpoint in a trial including patients with diabetes.

Technology Overload: Addressing Site Challenges Of Digital Trials
Watch as speakers explore the experiences of sites conducting digital and hybrid trials, discussing the critical benefits of a change management strategy and a unified digital trial platform.
