
TRIAL MANAGEMENT
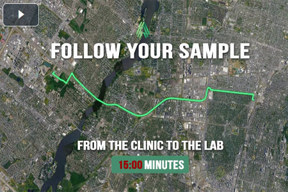
Accelerate Your Data With Our Co-Located Clinic And Lab
Watch as we take your fresh sample from our clinic to PBMC separation. This video overviews our optimized workflows that ensure timely processing, transport, and analysis of your samples.

Going From Good To Great As A TMF Champion
Gain insight into regulatory nuances, best practices for documentation management, and strategies for fostering a culture of compliance within the team.

Perceptive eClinical
Learn about a randomization and trial supply management (RTSM) solution that helps keep your trial on track.
At The Crossroads Of Data: eCOA And Sensors Converging For Novel Insights
Leveraging sensors and eCOA addresses increasing clinical trial complexity and ensures a more efficient and effective study design.
How RWD Reshapes Patient Recruitment Strategies
Discover how real-world data (RWD) is transforming patient recruitment by shifting from traditional site-centered methods to a patient-first approach.

Investigator SmartSelect
This advanced recommendation engine identifies protocol-specific investigators in just minutes, dramatically shortening a process that typically takes four to six weeks.

Yes, Your Study Is At Risk: Risk Mitigation Plans For Endpoints
Ocular endpoints often introduce unique bottlenecks that can stall enrollment and compromise data integrity. This presentation provides the tools for a streamlined, resilient clinical trial strategy.

The Golden Era Of GLP-1 Drugs
As GLP-1 drugs reshape the market, understanding their potential risks, applications, and economic effects is crucial for professionals in the pharmaceutical and healthcare industries.
Sponsors' Advice On Disclosure As A Service
Representatives from Teva and Lung Biotechnology share their insights for other sponsors considering Disclosure as a Service. Watch the short video now.

Clinical Research Without Borders
Explore strategies for navigating global clinical trials with, Afshawn Chakamian of Avidity Biosciences, Dr. Amy Raymond of Worldwide Clinical Trials, Scott Schliebner of Novotech, and Scout’s KimberLee Heidmann.

Practical Approaches To Faster Study Start-Ups
Learn how teams can leverage organizational, procedural, and technological solutions to recapture valuable days, weeks, and even months in the activation timelines of research sites.

Are Resource Constraints Impacting Your TMF Health?
Looking to quickly expand internal TMF resources with our anytime, anywhere TMF expertise? Unlock the potential to seamlessly access specialized TMF knowledge and support by viewing this presentation.

Take A Look Inside The Pharmacodynamic Toolbox
This session explores the flexible selection of pharmacodynamic measures to enhance the pharmacology, safety, and efficacy evaluation of a CNS-active drug in early clinical trials.

Learning About The Sensors In myMedidata
Learn how sensor data and advanced analytics can transform clinical research and enable your study to overcome data challenges, improving patient care.

Build Your Diversity Action Plan With AI And Patient Insights
Discover how AI and patient insights can help you build a compliant Diversity Action Plan and overcome barriers to achieve diverse clinical trial enrollment.

Modernizing Clinical Research With Patient-Centric Digital Data Collection
Accelerate clinical trial modernization with streamlined collection of continuous, high-precision data centered on the patient.

Say It With Technology: How Alnylam Fosters A Connected Relationship With Sites
Alnylam Pharmaceuticals is developing an entirely new class of medicines and delivered the world’s first and only approved RNAi therapeutics in 2018 and 2019. Learn how Alnylam earned its reputation among investigators as a sponsor of choice by standardizing studies...

Ensuring Completeness, Accuracy, And Consistency From Source To Archive
Discover how proficient TMF handling can proactively address potential pitfalls, fostering a state of preparedness for regulatory evaluations.

Navigating Early Phase CNS-Active Drug Development
Early development of CNS-active drugs requires in-depth clinical expertise. Altasciences’ Dr. Beatrice Setnik, Chief Scientific Officer, examines the strategies to ensure early comprehensive data collection to fulfill regulatory requirements and aid in evaluating th...

Solving The Impossible In Glioblastoma With An AI Synthetic Control Arm
This video discusses Medicenna’s MDNA55-05 clinical trial; a single-arm, open-label, multicenter study that offers a promising opportunity to improve outcomes for recurrent glioblastoma.

What Is The Secret To Ongoing TMF Health?
Delve into specific tactics clinical teams can utilize for establishing immediate and long-lasting improvements to their TMF business processes.

Think U.S.A.: The Operations Behind Your Clinical Trials
Ready to think differently about early-phase clinical development? Check out the spotlight on our U.S. clinical facilities and the incredible teams behind them.

CTIS Master Series 1: Are You Ready For CTIS?
In this informative webinar, clinical trial disclosure experts Francine Lane and Thomas Wicks provide a high-level overview of EU CTR/CTIS regulations, show how they differ from the EU CTD directive, share timelines for compliance, and discuss the scope of data sharing.

Optimize Protocols For More Successful Trials
Find out how you can harness the power of AI and world-class data to recommend endpoints and inclusion/exclusion criteria for streamlined protocol design that results in more positive trial outcomes.
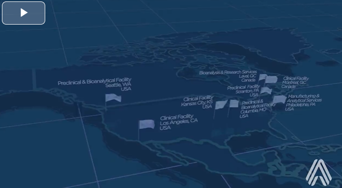
Altasciences Has You Covered From Coast To Coast
With your success in mind, we designed an integrated early phase drug development offering with eight locations, to get you from lead candidate selection to clinical proof of concept, and beyond.

Keeping Your Trials In Motion And Use AI Where It Makes Sense
Explore the "pinball" approach used by the Judi platform that is giving stakeholders a distinct competitive advantage in the clinical trial landscape.

TMF Management In Clinical Trials
Elevate your knowledge of clinical trials and the Trial Master File by exploring three critical topics.
Webinar: Reimbursement Methods For Sustainable CGT Business Models
Learn more about the key features of successful innovative pricing and reimbursement models as well as outcomes-based agreements and the ways to advance those strategies for cell and gene therapies.

Cognitive, Pharmacodynamic Testing During FIH CNS Trials
A comprehensive review of optimal timing and methodologies for cognitive and pharmacodynamic testing during first-in-human trials on CNS-active compounds.
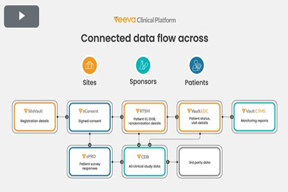
End-To-End Clinical Data Flow
Explore the interconnected data flow across Veeva Vault EDC, Veeva CDB, Veeva RTSM, and Veeva ePRO to visualize how these products work together seamlessly.
