
DATA MANAGEMENT
The Importance Of Reliable Data To Clinical Trial Activation
Find an easier way to identify and enroll eligible patients for your clinical trials with quality data and site selection.

Keeping Your Trials In Motion And Use AI Where It Makes Sense
Explore the "pinball" approach used by the Judi platform that is giving stakeholders a distinct competitive advantage in the clinical trial landscape.
Utilizing Curated Data To Boost Your Urology Research And Innovation
Obtain robust data from a reputable source to boost your urology research and propel your life science company's innovation in the urology space.
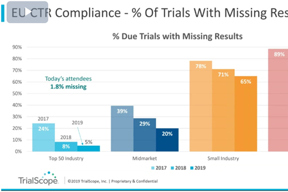
Disclosure & Transparency Trends Webinar
Hear results from TrialScope’s 2019 Global Clinical Trial Disclosure & Transparency Benchmark Survey, highlights from our Plain Language Summaries Survey, and predictions for 2020.

Pairing AI With High-Quality Data Leads To Clinical Success
While artificial intelligence (AI) is already impacting clinical trial planning, pairing AI with trusted data makes it even more powerful. Experts reveal how AI is being used today across feasibility, site selection, and trial strategy.

Manage Exponential Data Growth With Medidata Clinical Data Studio
Bring study teams together like never before for total data quality oversight.

Combining De-Identified EHR And Claims Data
Learn how EHR+ Data can improve your understanding of the patient's journey in ophthalmology, urology, and neurology clinical trials.

Using Data To Accelerate Racial Diversity In US Clinical Trials
Historically, US clinical trials have been shown to recruit disproportionately large percentages of White patients, raising concerns about the generalizability of clinical trial results to underrepresented racial minority patient populations. Because of this, the FDA has ...

Advanced AI-Powered TMF Automation
Automate time-consuming manual processes with the industry’s only AI-assisted filing and indexing, increasing speed and quality.
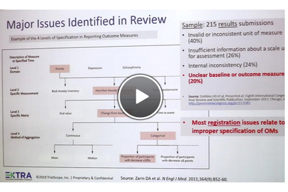
Improving The Quality Of Submitted Studies To ClinicalTrials.gov
Want to reduce QC comments? Hear tips from Dr. Deborah Zarin, former director of ClinicalTrials.gov, now with the MRCT Center. Dr. Zarin was keynote speaker at the recent EXTRA: TrialScope Transparency Experience.

TRACON: Reducing Clinical Trial Cost By Connecting Systems
Companies are gaining operational efficiencies with a single platform across R&D including CTMS, EDC, and Safety. Hear how one biotech is breaking down system silos and eliminating data reconciliation, reducing clinical trial costs by 70%.

Medidata Research Alliance
The Medidata Research Alliance collaborates with scientific and medical research communities to use clinical trial data for medical advancements and increased patient access to innovative treatments.

Keys To Successful Clinical Trial Data Handling And Re-use
In this video, Matt DeFranco, TrialScope Director of Solution Engineering, explains ETL (extract, transform, load) and API (application program interface). He then provides two case studies on their use in clinical trial disclosure and patient engagement.

Make Sense Of Unstructured Data In Neurology, Urology, and Ophthalmology
Transform your data and, ultimately, patient outcomes across three major therapeutic areas: Neurology, Urology, and Ophthalmology.

Clinical Data Studio: A Single Data Review Platform Fostering Unification Across Groups
This end-to-end digital review platform can assist both your risk management team and data management team, improving speed and facilitating database lock times.

Navigating Trials With Confidence: Building A Robust External Control Arm
Explore methods to build scientifically rigorous comparator groups to allow more patients access to life-saving treatments, accelerate trial timelines, and maximize resources.
Providing Rich Quality Data In The Neurology Space
From clinical development to post-approval, we provide quality insights powered by the Axon Registry through our exclusive partnership with the American Academy of Neurology.

Amplifying Evidence With Unified Clinical Trial Data Collection
In this presentation, we explore the transformative potential of integrating a unified clinical trial data collection platform with intelligent automation, challenging the inadequacies of the status quo.

Qdata From Verana Health For HEOR And Medical Affairs
Obtain real-world data on patient demographics, treatment patterns, and outcomes to help inform your future business decisions.

Streamline The Clinical Trial Disclosure Process
Core Data simplifies the registry submission and approval process by consolidating data for multiple registries into one single form, promoting consistency across registries and saving time by minimizing data entry.

Financial Scenario Planning — Budget With Confidence
Find out how financial scenario planning can revolutionize clinical trial budgeting with dynamic models, real-time data, and instant scenario visualization—all in one intuitive platform.

Pharmaprojects By Citeline
With Pharmaprojects, leverage your mastery of the R&D space to create winning strategies, identify the right drugs to license, and support the key decisions that will drive your company forward.

psiXchange: Intelligent, Automated Safety Reporting
Say goodbye to manual burdens and embrace efficiency by fully automating your safety reporting. This will significantly cut down on effort and costs, all while enhancing compliance standards.
Leveraging DHTs To Improve Data Collection And Analysis
How can wearable DHTs be leveraged to measure physical activity in patients with immunological diseases, enhancing clinical trials and advancing drug development?

Citeline Study Feasibility
This predictive analytic solution helps sponsors select global study sites to successfully enroll more patients and accelerate clinical trial cycle times. Study Feasibility leverages both human expertise and machine-learning algorithms trained on Citeline’s best-in-...

Trends In Clinical Transparency Policies
More and more sponsors are making their clinical trial transparency policy publicly available, says TrialScope Chief Strategy Officer Thomas Wicks. Hear additional disclosure and transparency trends.

Here To Help You Succeed
IQVIA Biotech is a standalone business of IQVIA with dedicated teams delivering clinical development solutions for biotech and emerging biopharma customers. Discover why IQVIA Biotech is the CRO partner of choice for biotech companies around the world.

Keeping Up With ClinOps: Why And How To Improve Delivery
Industry experts discuss the concept of a trial platform as a service and how it can accelerate and enhance the day-to-day functions of clinical operations throughout the entire lifecycle of a study.

A Framework For Selecting Reliable Clinical Endpoints From Real-World DHT Data
Dr. Andy Liu presents a case study determining which digital measure of physical activity would be a well-defined and reliable clinical endpoint in a trial including patients with diabetes.

Importance And Requirements Of Study Results Posting
In addition to laws requiring disclosure of clinical trial results, the public also demands this information. TrialScope Chief Strategy Officer Thomas Wicks explains why sponsors should share study results.
