Addressing The Top 7 Challenges In Decentralized Dermatology Trials
By Jasmina Jankicevic, Stacy Weil and Sonja VanWye
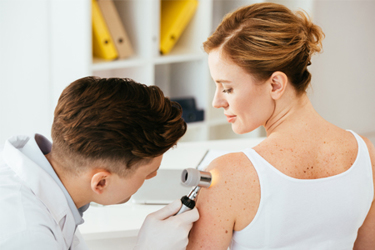
Each year, nearly 85 million Americans see a physician for at least one skin disease, with $75 billion in direct costs to the U.S. healthcare system.[i] While the FDA has approved more than 110 dermatology drugs, these treatments address only about 30 indications, leaving the vast majority of known skin conditions unaddressed. In recent years, there has been a significant increase in dermatology drug development to help address this need for safe, effective treatments for a broader swath of skin diseases. This trend—in conjunction with the push for more patient-focused studies and the pressures posed by the pandemic—has led to wider adoption of decentralized clinical trial approaches in dermatology.
In this blog post, we explore the top seven challenges in decentralized dermatology trials and how to address them.
Get unlimited access to:
Enter your credentials below to log in. Not yet a member of Clinical Leader? Subscribe today.
