Advancing Innovative Therapies For Mild-To-Moderate Psoriasis With Novel PASI-HD Assessment
By Kim A Papp, MD, Ph.D. FRCPC, president, Probity Medical Research
The Psoriasis Area and Severity Index, or PASI, was developed in the 1970s as a tool for assessing the severity of psoriatic lesions for the purpose of assessing outcomes in a psoriasis clinical development program. Over the past 50 years, PASI has become the most common clinical measure used to assess disease severity and response to therapy for people living with plaque psoriasis.
PASI is widely used despite significant limitations for patients with lower disease burden.
Despite its widespread use in clinical trials and clinical practice, PASI has several limitations that reduce its utility. As a tool initially developed to assess patients with severe psoriasis, it fails to meet the needs of people living with mild or moderate disease, who comprise most psoriasis patients. While it’s reasonable to question how a tool that optimally serves a minority of patients became the standard approach for assessing the severity of psoriasis in all patients, it’s important to recognize that at the time PASI was developed, handheld calculators were just entering the market and slide rules were still used for mathematical calculations. Consequently, PASI was intentionally designed for minimal mathematical complexity.
With PASI, the extent of psoriatic involvement is graded on a 0-6 scale, with a grade of 1 assigned for patients with body surface area (BSA) involvement of 1% to <10% within an anatomical region. This approach thus assigns a non-granular area score of 1 for body surface area (BSA) of less than 10%. A constant area score masks changes in disease extent, yet these changes may be very meaningful to patients. For example, a small plaque on the face may make patients extremely self-conscious about their appearance, which can negatively impact their social emotional health. Similarly, a small plaque on the genitals may interfere with patients’ sexuality and intimacy, which also can have psychosocial effects. These quality-of-life issues warrant significant concern regardless of overall disease severity or percent body surface area involved.
PASI-HD is designed to assess severity and therapeutic response in a less extensive disease.
With the goal of better meeting the needs of all patients living with psoriasis, I and others with expertise in treating patients with psoriasis and conducting clinical trials of novel psoriasis therapies have worked collaboratively with the clinical team at Arcutis Biotherapeutics to develop PASI-High Discrimination (PASI-HD). We intentionally designed PASI-HD using a more precise grading scale that provides a higher correlation with BSA compared with the traditional PASI.1 When using PASI-HD, areas of involvement in <10% of an anatomical region are given a score of 0.1 to 0.9 rather than being assigned a uniform score of 1. This allows identification of improvement or worsening in anatomical areas with <10% BSA involvement. Areas of involvement >10% are graded using the same scoring system as the traditional PASI.
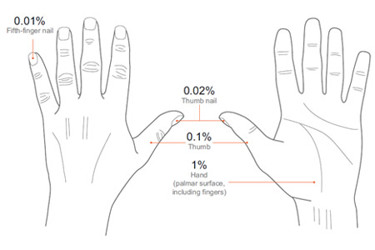
When assessing the severity of relatively small psoriatic plaques using PASI-HD, the affected area is estimated using the size of the patient’s hand as a relative standard. The palm of the hand along with the fingers is approximately 1% of total BSA. The thumb — from base to tip — is approximately 0.1%, the thumb nail is 0.02%, and the pinky nail is 0.01%. These simple standards (Figure 1) make it easy for physicians to document disease severity in small lesions and also allow tracking of large or small changes within two decimal places. In addition to simplifying the clinical use of PASI-HD, this hand-based standard empowers patients to visualize and understand how the assessment works.
Data presented at the American Academy of Dermatology (AAD) 2023 Annual Meeting2 support the increased precision that PASI-HD provides when assessing severity in BSA <10%.
The power to improve outcomes for patients with mild-to-moderate psoriasis is in our hands.
The data presented at AAD 2023 highlight another important benefit of PASI-HD, which is that it is informative for all patients with psoriasis, regardless of disease severity. The availability of a single tool that is meaningful to all patients should help facilitate adoption of PASI-HD as the gold standard tool for assessing patients with mild to moderate psoriasis. All of us who treat people living with psoriasis know that approved therapies, while very often effective, are not a panacea. Some are not appropriate for use on sensitive parts of the body or can only be used for limited periods of time. We also know that even “small” plaques can have big effects on patients’ quality of life and experience of their disease. PASI-HD has an important role to play in resolving these long-standing obstacles to improved patient care and outcomes.
From a therapeutic development standpoint, PASI-HD has the potential to discriminate positive treatment effects for plaques with <10% BSA involvement that are obscured with PASI. Additionally, the PASI-HD will better reflect the true size of the effect of treatment in the population of patients having lower area of disease involvement. Uncovering these responses has the potential to increase the overall success rate of investigational psoriasis therapies in clinical trials, which may help to speed approval of new treatment options that address unmet medical needs. By providing a more accurate assessment of therapeutic response, PASI-HD may also enable innovators of these novel therapies to make critical go/no-go decisions earlier in clinical development, reducing the time and money spent on candidates with low chances of success and allowing focused investment on novel therapies that are most likely to demonstrate significant benefit in robust clinical trials.
PASI-HD also has the potential to enhance our patients’ understanding of their own disease while allowing treatment decisions to be made and communicated using quantitative data. With PASI, physicians and patients are artificially forced to accept the idea that all plaques comprising <10% BSA are qualitatively equal, and that changes in BSA <10% are quantitatively meaningless. Regardless of what the numbers tell us, we know from experience that this is patently false. PASI-HD empowers physicians and patients with accurate and quantitative data. For example, when a patient comes in for a visit, I can tell them what their PASI-HD score is on that day, what it was at their last visit, and clearly define if their condition is the same, improving, or worsening. This quantitative approach can better help patients understand a recommendation to stay on their current therapy or to switch to a different treatment option. This simply isn’t possible with PASI, under which patients can be scored as “mild” or “moderate” visit after visit. This can create a false understanding that their treatment isn’t working, which may be a barrier to adhering to therapy.
Overcoming that barrier is, quite literally, in our hands. Using the PASI-HD hand standard to assess plaques and quantitate changes involving <10% BSA is simple and takes just a few seconds more than a traditional PASI assessment. With these minor changes to our traditional PASI routine, we have a single tool to quantify disease status and therapeutic response for all our patients. For those of us who treat people living with psoriasis, a “hands on” approach to patient care has never been more important.
About The Author:
 Dr. Kim Papp is a member of the College of Physicians and Surgeons of Ontario, a fellow of the Royal College of Physicians and Surgeons of Canada, and a fellow of the American Academy of Dermatology. The Waterloo, Ontario, Canada-based dermatologist has over 25 years’ experience as a principal investigator and has conducted over 170 psoriasis studies in which he closely supervised and assessed over 2,750 subjects. Dr. Papp is an internationally renowned key opinion leader in clinical research who conducts clinical trials on a wide range of dermatological disorders.
Dr. Kim Papp is a member of the College of Physicians and Surgeons of Ontario, a fellow of the Royal College of Physicians and Surgeons of Canada, and a fellow of the American Academy of Dermatology. The Waterloo, Ontario, Canada-based dermatologist has over 25 years’ experience as a principal investigator and has conducted over 170 psoriasis studies in which he closely supervised and assessed over 2,750 subjects. Dr. Papp is an internationally renowned key opinion leader in clinical research who conducts clinical trials on a wide range of dermatological disorders.
Dr. Papp, with the support of Probity Medical Research, an organization for which he serves as founder and president, has earned the distinction of top-enrolling investigator in over 70 international dermatology studies, is the author of over 275 publications, and is a highly sought-after speaker.
