Age Restrictions In Clinical Trials Unjustly Harm Adults With Childhood Cancer
By Rosemary Sherrod
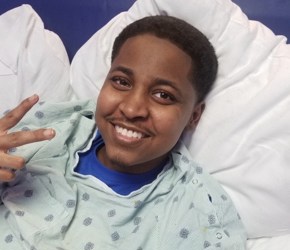
My son was 8 years old when he was first diagnosed with neuroblastoma, a cancer usually seen in young children. Over the next 15 years, we endured five heartbreaking recurrences. He was in and out of remission, his body a battlefield, and our hope constantly tested. Each time the cancer returned, we stood up to fight. But nothing prepared us for the devastation when, at age 21, the cancer returned — this time spreading to his brain and spine. We were more desperate than ever. A neurosurgeon had to place a shunt in QeVonte’s head after discovering one of the tumors at the base of his brain that was blocking fluid and causing buildup. After successfully performing the surgery to redirect the fluid into his stomach, the doctor gave us a devastating prognosis — QeVonte had approximately six months to live.
This experience is not isolated. Adolescents and young adults (AYAs), especially those with childhood-onset cancers, represent one of the most underserved groups in oncology today. They fall between two systems that rarely speak to one another, and many, like my son, are left without options when they need them most.
Still, we held onto hope. I took him to MD Anderson Cancer Center, where doctors joined our fight and searched tirelessly for clinical trial options. We finally found one — a promising experimental therapy in Indiana that specifically targeted neuroblastoma in the spine. I truly believed this was the miracle we had been hoping and praying for. But once again, we hit a wall. Despite meeting all medical criteria, my son was turned down. The reason? He was "too old." The trial's age cap excluded patients over 21 years old. This news was devastating for our entire family.
This raises the question: When does someone become too old for the cancer they’ve been fighting since childhood? My son had battled neuroblastoma since age 8. His doctor fought for him with everything he had. But it wasn’t QeVonte’s disease or determination that stood in the way — it was his age. 
The Hidden Survivors: Childhood Cancer Into Adulthood
Neuroblastoma is a rare pediatric cancer, with approximately 700 to 800 cases diagnosed annually in the U.S., mostly in children under 5 (American Cancer Society). My son was one of those cases who carried a childhood cancer into adulthood. But he's not alone. As of 2020, nearly 496,000 people in the U.S. were survivors of cancer first diagnosed before age 20 (National Cancer Institute), and that number has since grown. Today, more than half a million childhood cancer survivors live among us, many of them now in their 20s and 30s.
And the battle doesn’t end with remission. More than 95% of childhood cancer survivors will develop chronic health conditions by age 45, including heart issues, second cancers, or cognitive impairment (Children’s Oncology Group). Some experience relapses, while others develop treatment-related complications or second cancers. The risks don’t disappear when the patient becomes an adult.
The AYA Gap: Too Old For Pediatric Trials, Too Rare For Adult Ones
Age cutoffs are often set for regulatory simplicity or based on where care is delivered, pediatric vs. adult hospitals, not because of fundamental differences in biology. But this approach overlooks diseases like neuroblastoma that don’t conform to age boxes.
AYA cancer patients — ages 15 to 39 — often fall into a dangerous gray zone in research and care. They are significantly less likely to be enrolled in clinical trials than any other age group. According to the National Cancer Institute, while over 60% of children under 15 enroll in clinical trials, less than 10% of AYA patients between 15 and 39 do (NCI, 2020). Why? Because pediatric trials typically cap eligibility at age 18 or 21, and adult trials are rarely designed with childhood-origin cancers in mind. As a result, AYA patients — many of whom are still fighting the same diseases they faced as children — often become invisible in the research and treatment pipeline (Journal of Clinical Oncology, 2019).
This was our reality. My son’s cancer hadn’t changed — only his age. The trial held real promise to extend his life. Even with a seasoned neuroblastoma specialist passionately advocating on his behalf, the answer was still no. We waited over a month, holding onto hope, only to be met with silence that eventually gave way to heartbreak. The disappointment and frustration were crushing.
Include Them, Don’t Exclude Them: A Call To Action
This isn’t just a flaw in one trial’s criteria. It’s a systemic problem, and one that is solvable. We need to rethink age restrictions in clinical trials, especially for childhood cancers that persist or recur in adulthood. Trials should be driven by disease biology, not arbitrary age limits.
Here’s what I believe needs to happen:
- Broaden Age Eligibility: If a trial targets neuroblastoma, anyone with neuroblastoma, regardless of age, should be considered. The FDA has already begun encouraging more inclusive eligibility criteria. The FDA has issued guidance encouraging modernized eligibility criteria, and programs like the Children’s Oncology Group’s AYA strategy are beginning to address this gap. But more alignment is needed between regulators, sponsors, and hospitals to make age-inclusive research a norm, not an exception.
- Bridge Pediatric and Adult Oncology: Establish AYA programs and foster cross-institutional collaborations that ensure seamless transitions from pediatric to adult care, so patients don’t fall through the cracks in research, treatment, or clinical trial access. Survivors need not only access to care but structured survivorship plans, mental health support, and long-term monitoring… none of which should disappear when a patient turns 18.
- Empower Pediatric Centers to Treat AYAs: Many children’s hospitals could safely treat young adults with pediatric cancers but are held back by institutional policies. Let them decide case-by-case.
- Improve Trial Design and Awareness: Increase funding for trials that span broader age ranges. Encourage sponsors to prioritize inclusion over exclusion.
No family should have to fight two battles — one against cancer and another against bureaucracy. When childhood cancer follows someone into adulthood, our response shouldn’t be to shut the door. It should be to extend a hand and say, "We still see you. We’re still fighting with you."
Because hope, like healing, should never have an age limit.
About The Author:
 Rosemary Sherrod is a health equity advocate, product strategist, and proud mother of three whose work focuses on improving access to care and clinical trials for underserved communities. She is the author of Believing in the Dark and a leader in patient storytelling and digital health engagement.
Rosemary Sherrod is a health equity advocate, product strategist, and proud mother of three whose work focuses on improving access to care and clinical trials for underserved communities. She is the author of Believing in the Dark and a leader in patient storytelling and digital health engagement.
