An Inside Look At Novo Nordisk's Disease Experience Expert Panel (DEEP) Model
By Charline Coquerel, Sherina Kuruvilla, and Lukas Eichmann, Novo Nordisk

Patient-centricity is not just a buzzword. At Novo Nordisk, we recognize that if we want to develop innovative treatments for patients living with chronic diseases, it is essential to form meaningful partnerships in which our patients’ expertise, knowledge, and experience are included and put into action. It was this radical shift in thinking that led to the creation of the Novo Nordisk Disease Experience Expert Panel (DEEP), a network of patient experts and advocates. This shared commitment to forming a true partnership, where patients are routinely included and guide every part of our care delivery model, immediately resonated and took off. Since the program launched in 2015, it has grown from 15 members to more than 120 members from over 20 countries across multiple therapeutic areas — and we are still growing.
Model Principles And Members
Like others in our industry, our approach is based on an evolving worldview where people with serious chronic diseases are no longer seen as passive recipients of healthcare, but as experts through experience. By sharing their valuable insights into what it is like to live with a chronic disease, they can help us improve how we conduct research and develop innovative new treatments. In turn, by partnering to co-create these processes and initiatives with patients, we commit to respecting their input, ensuring it is evaluated and implemented. Sometimes, honoring patient input means revising our assumptions and changing elements of our programs to achieve the best possible outcome.
To foster mutually beneficial partnerships with DEEP members, we developed a model guided by the European Patients’ Academy on Therapeutic Innovation (EUPATI) frameworks.1 Our DEEP members are divided into four categories (illustrated below). The members of our DEEP network are primarily patients and caregivers, but they also include other patient experts, such as representatives of patient organizations. The model also includes colleagues at Novo Nordisk living with a chronic disease. These different patient roles bring a unique perspective and thereby provide a rich and diverse array of expertise to shape our R&D activities.
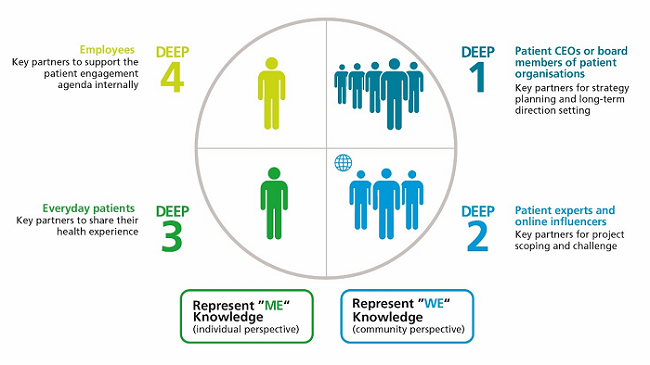
How The DEEP Model Works
When we engage DEEP members, we employ the DEEP CoCreate methodology, which defines the type of roles they may play and outlines a clear framework for gathering, reporting, and implementing insights. We always have a defined goal in mind to ensure that we are working together to solve a specific challenge.
Although the implementation is in a learning phase, we are working hard to embed patient partnership and co-creation into all key elements of what we do and make it an organic part of our processes. The DEEP network is engaged across many activities, including:
- DEEP Advisory Boards, where members are invited to provide input directly into key stages of the R&D value chain and co-create our processes and materials
- DEEP Summit, a two-day meeting of DEEP members and key Novo Nordisk colleagues to align, share best practices, and set the path ahead
- DEEPtalks, energetic 10-minute talks, also livestreamed on Facebook, where patients living with chronic diseases share their personal experiences
Often, more than one of these elements is activated for a given program, providing a comprehensive set of inputs.
The Model In Action: Co-creating A More Effective Obesity Research Program
Over the past few years, the DEEP network has been engaged to support the design and development of some of our clinical trial programs to ensure we design trials with the patient experience in mind. When we started planning our recent obesity clinical trial program, we had two common objectives in mind. First, obesity is complex, and we wanted to ensure that our research program was sensitive and accommodating to the needs of people living with obesity. Second, we had identified a number of potential trial challenges that we wanted to mitigate, including adherence to trial protocols, trial fatigue, and keeping participants motivated.
To get insights into how best to address these challenges and co-create solutions, the DEEP network was mobilized at several key stages, including:
- Panel discussion to provide our internal clinical trial team with an understanding of the journey of the patient with obesity
- Advisory boards on trial outline and trial design
- Workshops on developing patient recruitment and retention material
- Series of review meetings featuring a challenge and feedback session on informed consent and the healthy living support program
- Presentations at investigator meetings around the barriers and motivators when living with obesity
Perhaps one of the greatest things we learned from involving our DEEPs is that often the small changes can have the largest impact. When we heard one of our DEEP members from Germany tell us that, “Even going to the doctor is surprisingly difficult. We never know if the furniture in the waiting room or examination room will fit, or whether the medical equipment will be designed to work for a person whose size and weight are not the 'norm'…”, we immediately knew we had to take this into consideration. We informed our clinical trial teams that were responsible for performing the clinical trial feasibility visits to ensure that study centers were equipped to comfortably accommodate participants’ physical needs and able to address many other considerations raised.
Through all these activities we gained valuable insights that allowed us to adjust our approach and materials in a way that optimized the overall patient experience from the start.
Measuring The Impact Of The DEEP Model
As part of our desire to understand how participants feel about their involvement, check that we are delivering on our promise, and get feedback on how we can strive to do even better, we started sending quarterly surveys to the members of our DEEP network. As a result, we understand that 100 percent of those who responded would like to participate in DEEP activities in the future and 96 percent believe their activities make a positive impact on others. Collecting these metrics demonstrates the concrete benefits of patient engagement — for Novo Nordisk and for our DEEP members.
Building on our learnings, we are continuing to evolve the DEEP network. Our current top areas of focus are refining the DEEP CoCreate methodology to provide even more sophisticated and impactful outputs and including more diversity in our DEEP networks. This framework has proven a win-win situation, delivering tremendous value to Novo Nordisk and to the patients we partner with.
As an industry, we are aware that there is an increased focus on having the patient experience in mind, while delivering high-quality data when conducting our clinical trials. When we asked one of our DEEPs what would help keep them motivated to stay in our trials, the answer was simple: “If you commit to me, I will commit to you.” The real question from an industry perspective is: What more can we do to really listen to this advice, willingly learn from it, and truly commit to making a change?
References:
- The European Patients’ Academy (EUPATI). Guidance for patient involvement in industry-led medicines R&D. Available at: http://www.eupati.eu/patient-involvement/guidance-for-patient-involvement-in-industry-led-medicines-rd/. Accessed August 2019.
 About The Authors:
About The Authors:
Charline Coquerel is director and head of Global Patient Relations at Novo Nordisk. She is responsible for Novo Nordisk’s corporate patient engagement and advocacy strategy, ensuring there is a systematic approach for soliciting and integrating patients´ insights across the product development value-chain. She joined Novo Nordisk four years ago. Coquerel has over 16 years of industry experience in public affairs with a strong focus on advocacy and patient engagement, both at a global and local level.
 Sherina Kuruvilla is associate project director in the Global Patient Relations team at Novo Nordisk. She is currently responsible for leading the research and development (R&D) patient engagement strategy for Novo Nordisk. She joined Novo Nordisk in 2002. Kuruvilla has over 16 years of industry experience in clinical operations, while always having a strong focus on patient engagement and incorporating the patient voice in drug development.
Sherina Kuruvilla is associate project director in the Global Patient Relations team at Novo Nordisk. She is currently responsible for leading the research and development (R&D) patient engagement strategy for Novo Nordisk. She joined Novo Nordisk in 2002. Kuruvilla has over 16 years of industry experience in clinical operations, while always having a strong focus on patient engagement and incorporating the patient voice in drug development.
 Lukas Eichmann is associate patient relations manager in the Global Patient Relations team at Novo Nordisk. In his current role, he is managing the DEEP network and is responsible for analytics and implementation of insights, drawing on his experience with quantitative and qualitative analytics. He joined Novo Nordisk in 2018 and has previous experiences in his field of business administration and economics.
Lukas Eichmann is associate patient relations manager in the Global Patient Relations team at Novo Nordisk. In his current role, he is managing the DEEP network and is responsible for analytics and implementation of insights, drawing on his experience with quantitative and qualitative analytics. He joined Novo Nordisk in 2018 and has previous experiences in his field of business administration and economics.
