Best Practices For EDMS Implementation For Virtual & Early-Stage Pharmaceuticals
By Enith Morillo

Through the COVID-19 pandemic, companies pivoted and embraced remote work, establishing sustainable initiatives intended to remain in place well beyond returning to the office. With the boom of Zoom and telecommuting also came the rapid and somewhat uncontrolled adoption of electronic documentation management systems (EDMS).
There are numerous web-based, Part 11/Annex 11 compliant EDMS solutions available that provide a validated environment to manage controlled documentation such as standard operating procedures (SOPs), forms, work instructions, and templates and the corresponding change control process. These systems offer a virtual and accessible alternative to a traditional and notoriously hard to maintain paper-based system.
However, for virtual and early-stage pharmaceuticals with a remote workforce often comprising part-time consultants, an EDMS can pose several challenges when implemented too early in the product life cycle. These challenges range from incorrect configuration to slow adoption and can significantly detract from the advantages an EDMS purports. This article highlights the key factors virtual and early-stage pharmaceuticals should consider when evaluating the adoption of an EDMS to avoid the most common pitfalls.
Is An EDMS Appropriate For Your Organization?
The first step in EDMS adoption is determining whether the system is being considered as a solution to an existing problem, such as an inadequate document management process, or an improvement to a working, paper-based documentation process. In the former, the root cause of the problem may need to be identified and the process “fixed” prior to implementation of an EDMS, whereas in the latter, the EDMS is an improvement to a working process, increasing the chances of a successful deployment.
Virtual and early-stage pharmaceuticals heavily rely on contracted vendors for critical GXP activities, including manufacture of investigational drugs and clinical study conduct. In these companies, cross-functional teams are often tasked with building a comprehensive road map for adoption of technology solutions that includes budgetary considerations, timelines, resource requirements, and integration projections. An EDMS is one of the first solutions commonly planned for on this road map.
However, a key question to be asked is how many controlled documents need to be managed when most of the regulated activities are outsourced. Depending on the size of the organization, its outsourcing model, pipeline, and clinical stage, the phase-appropriate quality management system may consist of a relatively small number of controlled documents that complement the processes and procedures already in place at their partner CDMOs/CROs.
In such cases, adoption of an EDMS may not bring the return on investment expected and could consume resources that are best allocated to bring in-house high-impact, high-risk systems such as the electronic trial master file (eTMF) or safety database.
Selecting The Best Fit EDMS
As with any technology solution, choosing an EDMS that appropriately meets the needs of the organization is imperative for virtual and early-stage pharmaceuticals. Solutions designed to serve Big Pharma have the sophisticated level of complexity required to manage hundreds, if not thousands, of hierarchical, controlled documents across multiple locations globally. In these solutions, process flows account for numerous interdependencies, departments, job functions, and permission levels.
However, in a small company, an EDMS with that built-in complexity may create roadblocks to maintaining a streamlined documentation program, adding inefficiencies instead of removing them.
Thus, when evaluating EDMS vendors and their solutions, it is recommended to take a step-by-step approach that will result in selection of an optimal system for the organization. First in line, the vendor of the EDMS should be assessed in these four main categories, at minimum: maturity of the organization, quality of its technical support, caliber of its compliance, and focus of its leadership.
As for the EDMS, the first decision to be made is whether the system should be cloud-based, on-premises, or hybrid. This will naturally narrow the number of solutions. Next, EDMS candidates may be evaluated in relation to their security practices, integration capabilities with other systems, ability for the system to be customized and scaled up as the organization grows, searchability and reporting features, backup and restore, and availability of a sandbox environment, among others. Last, the system’s user-friendliness, mobility, and pricing structure can be looked at to further narrow the existing candidates. A Pugh chart like the one shown in Figure 1 may be a useful tool to aid the selection process, along with enlisting the support of a subject matter expert to vet the vendor, the system, and spearhead and oversee the validation process.
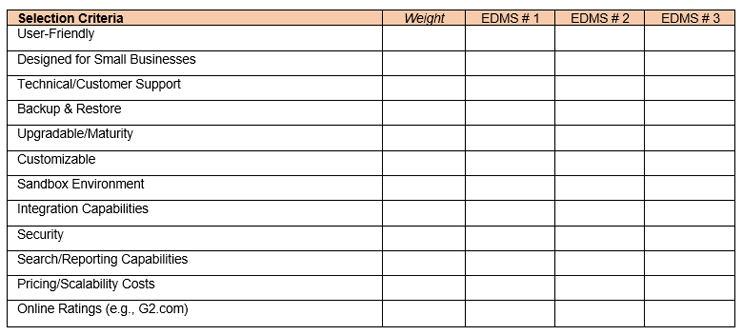
Correct Configuration Of The EDMS
Once an EDMS is selected and its vendor is qualified, some level of configuration may be required prior to validation. For web-based solutions, the configuration may be minimal, with workflows being simplified to reflect the size of the organization or document numbering being modified to match existing procedures.
However, when the functionality of the system is not well understood by the organization, significant errors may be made during the configuration phase that can wreak havoc downstream. For example, a common error that may occur is that of forcing file naming conventions in the system to align with existing document file names instead of maximizing the use of metadata.
Another challenge with configuration can arise when choosing what documentation is uploaded to the system. Although the EDMS may be able to hold an infinite number of documents within numerous categories, it is crucial for all in the organization to understand that an EDMS is not intended to be a replacement for cloud storage and using it as such can be counterproductive.
A best practice prior to adoption and configuration of an EDMS is to create a high-level inventory of the documentation managed in the organization and identify what documents are to be transitioned into the system and which ones will “live” outside of it. A good rule of thumb is to classify which documents are stationary and not likely to change (e.g., memos) vs. those that are dynamic, with a life cycle of their own and necessitating a change control process. As an example, SOPs and work instructions are generally the first type of documents migrated to an EDMS.
By documenting the process of selecting which documents will be migrated into the EDMS, the organization will in turn make an intentional decision on its use and more closely understand what may need configuration to increase the adoption and efficiency of the system.
Engaging Super Users
Wearing multiple hats is the norm in virtual and early-stage pharmaceuticals, so they are not likely to have a dedicated resource for EDMS administration. Therefore, system administration generally becomes a shared responsibility among the “super users” and tech savvy, who are often tasked with playing tech support for users who get locked out of the system, need to reset their electronic signature, or forget where to click during a change control workflow.
Although training sessions may be offered by the EDMS vendor, along with learning reference tools, these are generally not sufficient or comprehensive enough to ensure adequate and efficient system adoption. Lack of training and comfort with the system can lead to other challenges, such as users by-passing the system or using it incorrectly.
Therefore, involving the super users from the beginning is crucial to ensure they can champion the EDMS internally and be a resource to others struggling to embrace the system and its full functionality. Virtual and early-stage pharmaceuticals should consider system administration when evaluating the adoption of an EDMS and build in the required resource for it when budgeting for the solution.
Key Takeaways
As virtual and early-stage pharmaceuticals move through the product life cycle, the need for an EDMS may be identified and justified. However, ensuring that the road map to implementation begins with a well-defined process and agreed-upon criteria for vetting the vendor and the system is crucial for selecting the optimal system for the organization. Enlisting the support of a subject matter expert to weigh in the EDMS selection process, qualify the vendor, and support the configuration and validation of the system will help ensure that the EDMS meets the needs of your organization, is compliant, and is adequately implemented. Lastly, planning for a full- or part-time resource to administrate the system and support users during the adoption phase can only increase the return on investment and buy-in of the system when your organization is ready for it.
About The Author:
 Enith Morillo, M.Sc., is the founder, president, and principal consultant of Cadoret Global, a quality and compliance consulting firm that specializes in supporting virtual, early-stage, and small pharmaceuticals in taking their investigational drug through development and into Phase 1-2 clinical trials. Cadoret Global is a woman-owned and minority-owned certified company and an alumni of the Goldman Sachs 10,000 small businesses program. She can be reached at Enith.Morillo@CadoretGlobal.com or on LinkedIn.
Enith Morillo, M.Sc., is the founder, president, and principal consultant of Cadoret Global, a quality and compliance consulting firm that specializes in supporting virtual, early-stage, and small pharmaceuticals in taking their investigational drug through development and into Phase 1-2 clinical trials. Cadoret Global is a woman-owned and minority-owned certified company and an alumni of the Goldman Sachs 10,000 small businesses program. She can be reached at Enith.Morillo@CadoretGlobal.com or on LinkedIn.
