7 Breakthrough Behaviors For Clinical Research Project Managers
By Dalfoni Banerjee, principal consultant and CEO, 3Sixty Pharma Solutions LLC

Clinical project managers, your “firefighting” days can be over if you internalize and demonstrate these seven indispensable behaviors.
During my 25+ years in the pharmaceutical Industry, with over 20 of those devoted to project and program management, I’ve seen many projects fall short of target goals — frankly, including some of my own in the early years. Often, the root cause of a project’s derailment is the absence of what I call “visionary concepts” that improve the likelihood of success. Visionary concepts are not to be confused with “processes,” whether well-defined or not: Whereas processes define how to achieve the goal within the organization’s framework, visionary concepts define strategic and soft-skill behaviors for achieving goals, agnostic of process or organizational framework.
For clinical trial project managers (CTPMs), there are core clinical knowledge requirements across organizations: therapeutic, regulatory, and clinical trial components and goals. However, expertise in strategy is not a prerequisite, and neither are some important soft skills that are foundational for CTPM success.
In this article, I’ll discuss seven visionary concepts, essential behaviors that, if employed (i.e., internalized and demonstrated), can go a long way in obviating the need for CTPMs to spend significant time firefighting.
Additionally, since learning by example can be quite powerful, I’ll highlight some of the pitfalls I’ve experienced. I’ll also outline techniques I’ve employed to avoid those pitfalls and, importantly, some hefty benefits garnered when the visionary concepts have been employed.
The Case For Change: Embracing And Demonstrating Visionary Concepts
CTPMs confront a myriad of tactical challenges, which can consume nearly 100 percent of a CTPM’s working hours. Therefore, many CTPMs rarely, if ever, have the luxury of stepping back and envisioning trial conduct from a birds-eye view. Timelines are always too tight, and other priorities tend to predominate. Indeed, CTPMs would be grateful for the opportunity to connect various elements and form a cohesive and seamless process continuum that has all the bells and whistles. Oftentimes, instead, the CTPM finds herself devoting the majority of, if not all, effort working through a series of steps based on SOPs and regulatory, company, and industry statutes and guidelines — as shown in Figure 1 – and putting out fires.
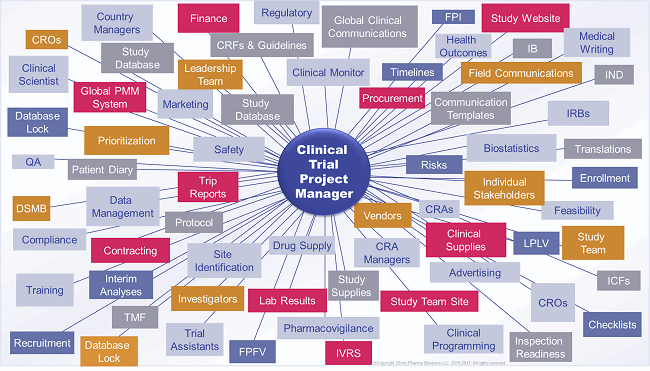
Figure 1: The clinical trial project manager is the hub of a study. (Graphic is representative, not all-inclusive.)
Adherence to all of the above is vital — no question about it. However, seven essential behaviors can bolster the success of CTPMs, while allowing for operation within company and regulatory edicts — and ultimately reinforce an organization’s efforts to advance patient care via happy CTPMs and efficiently-run clinical trials.
- Plan, Organize, Prioritize, and Establish Metrics
- Infuse Customer Focus
- Innovate
- Remain Goal-Focused
- Communicate with Savvy
- Email Like a Pro
- Plan for the Unexpected
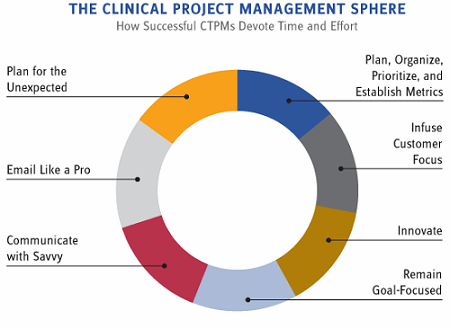
Figure 2: The clinical project management sphere
1. Plan, Organize, Prioritize, And Establish Metrics
Everybody knows planning, organizing, prioritizing, and establishing metrics are the right things to do. However, they easily fall by the wayside in the fog of running a clinical study or program. So, how do you keep them front and center and make sure they happen?
Schedule time to think! Add daily “thinking time” to your calendar and guard it jealously. Use your “thinking time” to keep a handle on the things you know are necessary for project success: establishing project scope, refining objectives, defining the course of action required to attain the objectives, and otherwise planning, organizing, prioritizing, and establishing metrics. Remember, failing to plan is planning to fail.
Whereas none of these things can be done in a vacuum, as the project lead, it is incumbent upon you to do some of the heavy lifting.
Tip: Checklists are essential, and I use them throughout each project’s life cycle — leveraging existing ones when I can (Google is a fine tool) or developing them myself, if necessary.
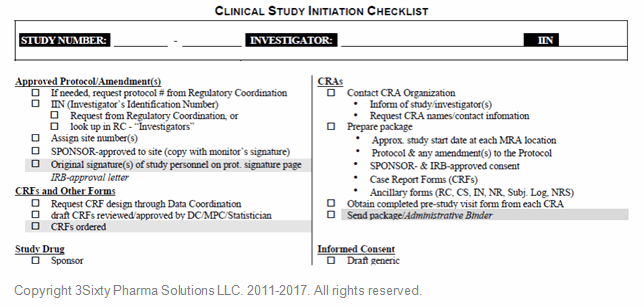
Figure 3: Clinical study initiation checklist
2. Infuse Customer Focus
Just so we are on the same page, but at the risk of stating the obvious, let’s begin with a definition of “customer focus.” According to the Cambridge Dictionaries Online, customer focus is defined as “paying great attention to the needs and opinions of customers.” Business Dictionary adds another layer, stating customer focus is “the orientation of an organization toward serving its clients' needs. Having a customer focus is usually a strong contributor to the overall success of a business and involves ensuring that all aspects of the company put its customers' satisfaction first.”
Now that we are aligned on what customer focus means, who are the CTPM’s customers? To help ensure the success of clinical studies, all study stakeholders are customers of the CTPM. Every project team member, every investigator, all field personnel, CROs, vendors — everyone who has a role in the conduct of the study.
Sounds like an enormous job. How can “customer-focused” be made a reality while conducting clinical trials? By engendering ownership, responsibility, clarity, and transparency — and making it as easy as possible for each stakeholder to succeed. It’s essential that a CTPM understands what will be required (both functionally and emotionally) for each stakeholder to succeed and how to ensure the requirements are met — or, better yet, empowering the stakeholder to do so.
I’ll give you an example from my experience preparing for, and executing, an investigator meeting (IM). Recognizing that:
- IM attendees sometimes are not as interested in the content as we would like them to be.
- The IM presenters’ day jobs are usually not dependent on them being engaging presenters.
- The success of the trial is highly dependent on attendee engagement (during the IM and beyond).
In-house trainers were engaged to deliver a 1.5-hour “presentation skills primer” to IM presenters. Additionally, the trainers agreed to offer one-on-one coaching afterward. All presenters attended the training, and 99 percent took advantage of the coaching.
Outcome: One presenter (who had been in dire need of enhanced presentation skills) received a standing ovation at the IM.
3. Innovate
The key to innovation is eliminating the following phrase from mind and vernacular: “because we’ve always done it this way.”
Some call it “thinking outside the box”. Recognizing the “box” in the clinical study arena is virtually airtight, still, it behooves the CTPM to approach the conduct of clinical trials creatively. In doing so, opportunities might surface that would have otherwise remained buried.
Here’s another example from my experience: We surveyed investigators, in part for ideas on how to best reach patients who might be appropriate for the study. Another reason was to get their opinions on what we, as the sponsor, could do to make it easier for them. (Remember most people like to share their opinions; investigators are no different.) Prototypes of the top ideas were available during the IM.
Outcome: Pediatric trials were fully enrolled six months early. In fact, the studies enrolled so quickly that only half of the planned sites participated because the other half were still getting up and running and had not been initiated.
4. Remain Goal-Focused
As a CTPM, you must be in the weeds enough to know the subject matter – what’s going on with each of the functions and stakeholders – but have the ability to rise above the weeds and focus on the objectives.
The key to remaining goal-focused is, throughout the project, defining, tracking, measuring, and re-evaluating:
- goals (I know it seems obvious, but you’d be surprised how often this slips through the cracks)
- project scope
- roles and responsibilities
- deliverables
- timelines
- key performance metrics and indicators
- risks and issues.
Tip: I’ve found the best way to ensure the CTPM keeps the project goals in mind is to frame all project activities — especially meetings and communications — around those goals using a road map. The road map is, essentially, a high-level timeline that includes dates, resources, and budget. This can be accomplished using project management software or a simple spreadsheet.
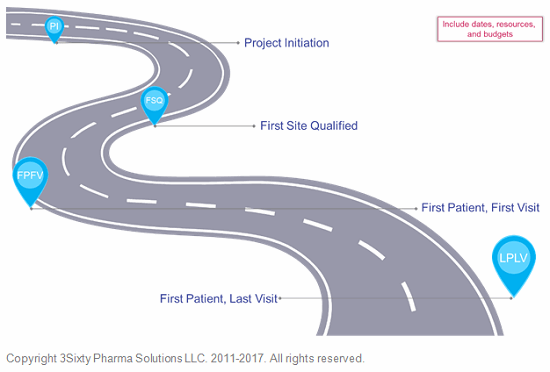
Figure 4: Project roadmap
5. Communicate with Savvy
“Seek first to understand, then to be understood” – Stephen Covey
You’ve heard it before – communication is key. And it’s true. I often remind clients that even when they are doing a stellar job, if they aren’t communicating with key stakeholders in ways that resonate with them, all their excellent work and successes are almost for naught.
Communication is about connection: Again, we must communicate with target audiences in ways that resonate.
This is how you do it:
- Build relationships.
- Understand the subject matter.
- Practice active listening – use a feedback loop.
- Pay attention to nonverbal signals.
- Define key stakeholders on the project.
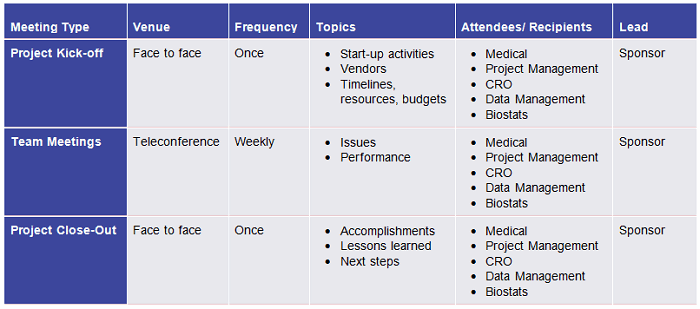
- Maintain a communication matrix (Figure 3).
- Conduct effective meetings. Ineffective meetings are a drag on teams. It is important to get this right. Here’s how:
- Invite only those who need to attend.
- Keep meetings focused on the road map and issues.
- Focus your agenda on goals related to various functions (e.g., clinical, CRO, data management, safety, regulatory).
- Create an agenda (high-level topics only) for every meeting. If there is no agenda, there should be no meeting.
- Send the agenda prior to the meeting.
- Include a placeholder for “any other business” (AOB).
- Capture action items during each meeting.
Active listening, speaking with clarity and purpose, facilitating quality live and virtual meetings, and reading people (being empathetic) are essential to communicating with savvy. I believe the metaphorical bow that ties all of those things together is communicating the customer’s WIIFM (what’s in it for me) and making it easy for your team members to do their jobs.
Table 1: Example Communication Matrix
6. Email Like a Pro
Conveying important information via email is challenging for busy teams. Important study-related information needs to be conveyed via email so the entire team will be aware immediately. Here’s a method for emailing like a pro:
- Email title is attention-getting: “Action Required by [Date]”
- Keep the email as brief as possible
- Begin the email with, “The purpose of this message is to provide information that will make things easier for you.”
- List the three topics that will be covered at the top of the message
- Include interactivity by
- Hyperlinking the topics
- Using the “voting” function for recipient acknowledgement of receipt
7. Plan for the Unexpected
A CTPM is a problem-solver, but, just as importantly, a problem-anticipator/risk-identifier and mitigator. As the saying goes, anything that can go wrong, will go wrong.
That seems like a tall order — how do you plan for the unexpected? Well, if you’ve diligently…
- planned, organized, prioritized, and established metrics
- infused customer focus
- innovated
- remained goal-focused
- communicated with savvy
- emailed like a pro
…you have set the course to minimize or eliminate firefighting and anticipate factors that could impact your project’s timeline, performance, or budget, thereby preventing the risk from becoming a reality — i.e., an issue that must be addressed.
Each CTPM’s situation is different, but the figure below provides guidance on percentages of time a CTPM should be devoted to each behavior, based on the combined experience of our consultants.
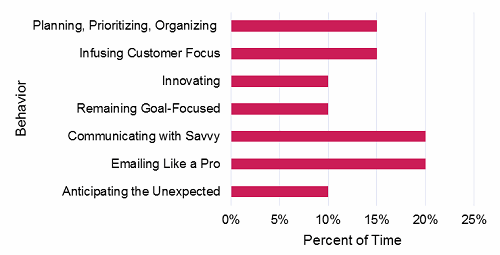
Figure 5: CTPM time allocation goals
Conclusion
Internalizing and demonstrating these seven behaviors will help CTPMs avoid firefighting and:
- Avoid or mitigate loss of:
- time; less-than-effective utilization of resources
- valuable threads that connect elements of the process
- opportunity to introduce value-added innovations
- information and insights
- efficiency and effectiveness.
- Prevent being overly focused on:
- tactics, rather than strategy
- tracking, rather than contingency planning and risk mitigation
- being reactive, rather than proactive
- the mind-set of “the way it has always been done,” rather than innovating
- mainly the study, rather than the customer.
With the seven essential behaviors, CTPMs will:
- deliver with excellence.
- run trials more efficiently and experience less burnout and frustration
- become highly strategic and oversee clinical trials that are more likely to successfully enroll on time/budget or early
- set long- and short-term goals
- prepare clear road maps to achieve goals
- maintain an awareness of the “big picture,” thereby being optimally effective
- continuously think about better ways to get things done, which leads to innovation
- garner and incorporate new expertise into daily routines
- be compelling communicators, goal-focused facilitators, persuasive and disciplined presenters, adept negotiators
- bolster their ability to positively contribute to advancing patient care.
For organizations, cultivating a framework that facilitates the embrace and demonstration of the seven behaviors can translate to CTPMs having greater job satisfaction and to retaining talented CTMs at a higher rate, which drives time, cost, and resource savings while meeting the ultimate goal — advancing patient care.
Good luck!
About The Author:
 Dalfoni Banerjee is an award-winning, solution-focused entrepreneur with over 25 years of pharmaceutical experience. She is the founder and CEO of the consulting firm 3Sixty Pharma Solutions, a certified woman-owned, minority-owned small business that partners with clients to add efficiency, innovation, and savings to business-critical projects — from clinical research through marketing and product launch. Her therapeutic specialties include immunology, biosimilars, vaccines, CNS, analgesia/pain, and ophthalmics, among others.
Dalfoni Banerjee is an award-winning, solution-focused entrepreneur with over 25 years of pharmaceutical experience. She is the founder and CEO of the consulting firm 3Sixty Pharma Solutions, a certified woman-owned, minority-owned small business that partners with clients to add efficiency, innovation, and savings to business-critical projects — from clinical research through marketing and product launch. Her therapeutic specialties include immunology, biosimilars, vaccines, CNS, analgesia/pain, and ophthalmics, among others.
Dalfoni is an invited speaker and moderator on a variety of clinical research, project management, and consulting topics, is a contributing author on several scientific publications, and has received 30+ awards.
