Can Better Project Management Be Your Trial's Secret Weapon?
By Life Science Connect Editorial Staff

It’s no great secret that 90% of clinical trials1 fail to yield a commercially approved drug. And while there are certainly failures of science at play, there are also myriad oversights in communication and planning. After a wide-ranging career across the clinical research space — including stints at Vanderbilt University Medical Center, IQVIA, and Pfizer — Jess Thompson, CEO of Clinical Research Pro, recognized the impact of implementing better project management to improve trial outcomes.
Thompson’s discovery of broadly applicable, evidence-based project management principles led her to launch Clinical Research Pro, an organization geared toward educating clinical research professionals on project management strategies that support trial success and mitigate burnout. In a recent Clinical Leader Live event, Project Management For All Clinical Research Professionals, Thompson highlighted why project management is pertinent across the clinical pipeline and how stakeholders can activate strategic approaches to promote the success of their drugs.
Hidden Project Management Tasks
Thompson described the fundamental turning point of her career: “Project management is the use of specific knowledge, skills, tools, and techniques to deliver something of value to people. I had been doing this work all along and I didn’t know it.” She also emphasized the sneaking suspicion that many other clinical research professionals are in the same boat, citing the following trial activities as project management tasks:
- Maintaining communication across multiple project teams
- Scheduling site initiation visits with monitors, investigators, and site staff
- Serving as the main point of contact between CRO, site, and sponsor
- Coordinating lab shipments, vendor deliverables, and drug supply with sites
- Managing project deliverables, budgets, and resources while tracking enrollment goals
- Tracking staff assignments across studies to keep others accountable for deadlines and quality
- Ensuring timely and accurate safety reporting
In many cases, clinical research team members may be “accidental project managers,” or those who conduct the tasks without the title. To distinguish those conducting project management, it is vital to define the differences between projects versus operations:
- Projects are temporary endeavors with defined start and end dates. They are change-focused, aimed at delivering something new and innovative. For example, a clinical trial is a project aimed at gathering data for drug approval.
- Operations are ongoing organizational functions. They are continuous, repetitive, and support day-to-day functionality. This includes performance reviews, SOP maintenance, and maintaining a CTMS.
Management of both project and operational tasks is essential, but for clinical trials, much of the work benefits from a project-management approach.
Balancing Risk While Keeping Trials On Track
To better explain how today’s trials are rife with risk, Thompson broke down a Pfizer study she worked on by approximate numbers: 321 sites; 45,789 participants; 460,152 sub-projects; and over 3,140 team members performing project-management duties. The astounding result was upwards of 4.9 million communication channels, underscoring the number of opportunities for missed deadlines, missed communication, and over- or under-estimated budgets. “We have so many things going on simultaneously that, without proper project management training, we run into operational issues,” noted Thompson. However, she noted that clinical teams can proactively keep their trials on track with cross-functional groups leveraging Project Management Institute approaches focused on value, iterative progress, and small, manageable cycles.
The Tenets Of An Effective Clinical Project Management Model
Implementing project management strategies across a clinical trial takes buy-in from the individual to company level. To succeed, stakeholders should be familiar with the core components of a clinical project-management model.
A Hybrid Approach
There are two common styles of project management: waterfall and agile. The waterfall model focuses on executing a plan in sequential order and consists of fixed requirements and deliverables, providing opportunities for feedback at the beginning and end of a cycle. Frequently implemented in tech, the agile model leverages short cycles and rapidly adapts based on feedback and changing requirements.
Clinical project management requires a hybrid of these two strategies, using the structured phases of waterfall planning alongside the flexibility of an agile model. With an adaptable mindset, clinical teams can focus on prioritizing the outcomes that matter most to patients, modifying recruitment strategies in real time, and embracing change as an opportunity for improvement. Trials teams should break trials into smaller, manageable cycles to secure faster feedback, implement frequent check-ins with sites to adapt studies based on real-world results, and maintain collaboration and transparency across teams.
A Project Management Plan
A project management plan (PMP) is the blueprint of a project, outlining how things will happen and when, who will oversee and pay for them, and the scope. It should serve as the central source of truth across stakeholders to ensure teams are aligned on timeline, cost, quality, risks, and communication plans to support the trial finishing on time. An aligned document enables consistency and ease of information transfer across departments.
Communication Management
With millions of potential communication channels at play, one rogue stakeholder could derail a project. Thus, trial project managers must identify early where stakeholders fall on the communication grid (Figure 1):
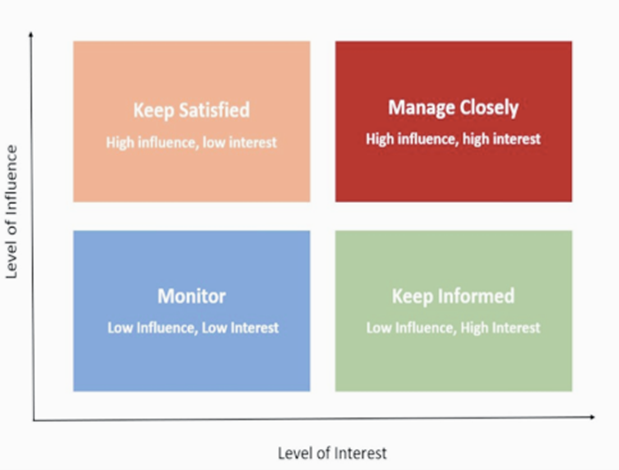
Figure 1. Project stakeholder communication framework based on relative influence and interest.
- High influence / high interest: This includes PIs and internal study teams. Their communication plan requires weekly status updates, regular team meetings, and recruitment updates.
- High influence / low interest: Think FDA, other regulators, and an IRB. These bodies make or break a drug’s success. Due dates and communication requirements should be tracked vigilantly.
- Low influence / high interest: Participants of the study. Their communication plan should be informed by the sponsor but might include regular newsletter updates on the trial’s progress.
- Low influence / low interest: This group consists of the public and broader medical community, who can be updated via news releases, interviews, and social media.
Once a trial’s stakeholders have been designated, project managers must determine how best to disseminate critical information and at what intervals.
Change Management
When it comes to change management, Thompson recommended the ADKAR approach, which she defined as “an individually focused framework that guides individuals and organizations through change by focusing on what drives adoption and sustainability.”
- (A)wareness: Educate stakeholders on why change is needed. For example, communicate why a new electronic patient recorded outcomes (ePRO) system will improve data quality.
- (D)esire: Foster a willingness to participate in change by demonstrating potential benefits to their role, i.e., showing sites how process standardization will reduce their workload.
- (K)nowledge: Provide training and information on how to implement the change, perhaps through a GCP refresher course.
- (A)bility: Ensure teams have the skills to implement change. Provide hands-on modules that reinforce knowledge.
- (R)einforcement: Support the change via monitoring, continuous support, and ongoing feedback loops.
This model strives to support team members across the implementation of protocol amendments, new technologies, and other major changes.
Risk Management
The ICH’s E6(R3) guidelines for GCP emphasize fit-for-purpose quality, calling on sponsors to leverage a systemic approach to risk-based quality management focused on participant safety and data integrity. Thompson outlined five critical steps in proactive risk management:
- Identify risks: Brainstorm across stakeholders to identify both positive and negative risks and write a comprehensive list.
- Assess risks: Create a ranking of the risks based on likelihood of occurrence and impact to the project if they do occur.
- Plan contingency responses: Determine risk response plans based on the company’s risk tolerance and associated costs. For negative risks, determine whether to escalate, avoid, transfer, mitigate, or accept. For positive risks, evaluate whether to escalate, exploit, enhance, or accept.
- Status reports: Conduct regular status reports to determine risk evolution and whether they need adapted contingency responses.
- Lessons learned: Track the successful strategies and missteps that arise throughout this process, helping teams to minimize these challenges in the future.
Plan Smarter, Not Harder
Baking project management best practices into the framework of a clinical trial could help significantly reduce risks, missteps, and miscommunications. To help mitigate the burnout of accidental project management, clinical trial teams should strive to embrace proven project-management strategies that foster flexibility and cohesion across stakeholders, leveraging efficient planning to illuminate a trial’s pathway to success.
References
- Duxin, S., Gao, W., Hu, H., Zhou, S. (2022). Why 90% of clinical drug development fails and how to improve it. Acta Pharmaceutica Sinica B. https://www.sciencedirect.com/science/article/pii/S2211383522000521?via%3Dihub
About Jess Thompson
 Jess Thompson is the founder of Clinical Research Pro, with over 15 years of hands-on clinical research experience spanning clinical laboratories, research sites, CROs, and pharmaceutical companies. Driven by her passion for empowering clinical research professionals, Jess established Clinical Research Pro to advance education, professional development, and career growth across the industry.
Jess Thompson is the founder of Clinical Research Pro, with over 15 years of hands-on clinical research experience spanning clinical laboratories, research sites, CROs, and pharmaceutical companies. Driven by her passion for empowering clinical research professionals, Jess established Clinical Research Pro to advance education, professional development, and career growth across the industry.
Under Jess’s leadership, Clinical Research Pro offers a supportive community that integrates professional development with wellness initiatives tailored to the unique challenges of clinical research. By emphasizing project management, risk management, change management, and leadership—alongside mental and physical well-being — Jess ensures that professionals are supported as whole individuals. Her deep industry knowledge, combined with a holistic approach to career and personal growth, allows Jess to deliver impactful training and resources that help clinical research professionals excel in both their work and their personal lives.
