Capacity & Competency Of The Clinical Research Workforce — Today & Tomorrow
By Beth Harper
It’s not simply an issue of supply and demand. It’s closer to life and death. Put bluntly, we may not be able to keep up with future clinical trial demand because our workforce isn’t growing fast enough. In fact, by some metrics, we’re already falling behind.
with future clinical trial demand because our workforce isn’t growing fast enough. In fact, by some metrics, we’re already falling behind.
The consequences are dire. We risk missing out on access to new drugs and therapies designed to mitigate suffering and prolong life. We risk slowing our amazing progress in oncology and other therapeutic areas. We risk losing ground in our battle against Alzheimer’s and related diseases.
Surveys and available data suggest that over the last three years there are signs that demand for clinical trial personnel has been increasing, with average compound annual growth in monthly job postings activity of 9.3 percent across all clinical research positions.1 While a nearly 10 percent growth in demand sounds robust, it is somewhat sobering when we see that the average year-over-year growth in clinical trials activities is 12.2 percent over the same time period.
The prognosis is grim: The clinical research workforce may not be keeping pace with current clinical trial workload needs. And there are obstacles to entering and thriving in the clinical trial workforce we must confront, repair, or remove. Beyond the capacity issue, many clinical research professionals are overwhelmed with the growing volume of technologies, tools, systems, and apps that require new or different competencies. While the technologies are driving needed changes in archaic processes, they also risk adding to trial complexity with a workforce that is already strained. And if that weren’t enough, the COVID-19 pandemic is accelerating a plethora of decentralized clinical trials (DCTs) and hybrid trial designs at a time where we are just grappling with the terms, concepts, and definitions, let alone ways to operationalize these novel approaches to making trials more patient-centric.
Fortunately, there is time to correct the future if we act today.
Growth In Use Of Technology Requires Commitment To Training
First, it’s critical to modernize our workforce with enhanced training in technology to improve their technical competencies. Use of technology in clinical research is increasing. According to the Tufts Center for the Study of Drug Development, “sponsors and CROs (contract research organizations) use approximately six applications to support each clinical study,” which is double what was used 10 years ago.2 Key applications of technology within clinical trials include systems for electronic informed consent (eConsent), electronic source documentations (eSource), electronic patient-reported outcomes (ePRO), electronic clinical outcome assessment (eCOA), patient portals, and site portals, as well as the introduction of wearables, sensors, connected devices, and telemedicine platforms. While some of these technologies were created to support remote work with the patient, there may be continued use even when the patient is on-site. The good news is that we can, in fact, teach people to become more proficient, but it takes organizational commitment, support from the technology vendors, and a willingness of the individuals to do so.3
Even with a more technically adept workforce, the volume and diversity of all the technology can also place additional burden on study sites. Often, the drug development industry fails to fully consider the impact to sites when doling out more technology. How much time is site staff investing in training subjects on various mobile devices? How are we enabling them to be effective troubleshooters when the subjects have issues? What type of compensation is appropriate to accommodate these and other factors? We need more collaboration between all stakeholders as new technology is introduced to help ease this burden.
Not only do we need to better train current and future practitioners when it comes to harnessing game-changing new technologies, we also need to create new roles with enhanced competencies to accommodate working in DCTs and hybrid clinical trials. ACRP recently convened a working group to better understand the impact of various trial models on the clinical research workforce as well as the different types of roles that are emerging to work in nontraditional environments.4 While the industry has not yet achieved consensus on the exact definitions, the working group differentiated between a fully decentralized trial (one that utilizes all technology aspects to their fullest extent) and a hybrid trial (one that combines some aspects of a DCT with those of a traditional clinical trial). The group found significant variation when designing hybrid studies, ranging from having all sites perform both traditional and decentralized processes to having one country work in a decentralized fashion while another country works in a traditional model. The group concluded that the majority of trials that implement aspects of decentralization will be hybrid trials, at least for the short term.
In evaluating the changing roles and competency requirements, the group also considered trials requiring extensive technologies as well as the impact of COVID-19, where many functions are being done remotely. Certainly, decentralized, or hybrid, trials were growing in popularity even before COVID-19 hit, but the emergency conditions created by the pandemic have absolutely accelerated adoption. Many sponsors/CROs and sites now have experience with various aspects of decentralization, and it is likely many future trials will include one or more of these parameters. Some sponsors are mandating that DCTs be considered for every protocol. Other sponsors are adding contingency options into their protocol templates to allow the use of technology (e.g., telemedicine) to facilitate trial procedures. Regardless, this increasing use of technology for all trials means that clinical research roles will need to evolve and operate differently to accommodate this new environment.
Evolving Roles Present New Challenges and Opportunities
The working group found that a number of new roles are evolving, both for roles that are patient-facing as well as those that are more supportive in nature, whether at the investigative site, CRO, or sponsor organization.
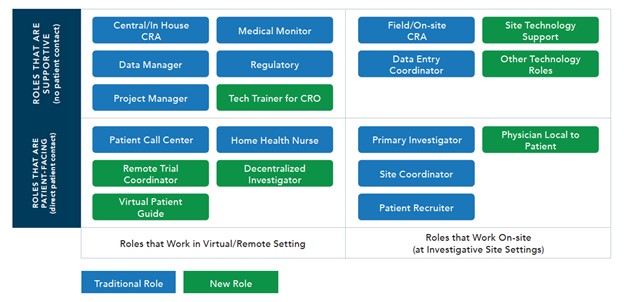
As noted in the graphic, the roles in green represent newer roles that are being created; however, the working group anticipates that all roles will continue to evolve and that many of the on-site roles will have a virtual component.
As roles evolve so, too, will clinical trial processes, although this wasn’t the focus of the working group’s research. One key takeaway from the research was that for sites, soft skills (flexibility and adaptability) will be the number one competency that needs to be developed. This highlights how important attitude is when working in an area that is changing so rapidly. Unsurprisingly, for non-site roles, clinical research professionals will need to develop strong technology skills ranging from learning video meeting functionality and etiquette, document sharing, data analysis, and specific trial technology (e.g., eConsent, eSource, ePRO) to learning how to support and troubleshoot trial-specific technology with the sites. As clinical research evolves, the successful professional will embrace change and be open to learning new technology and new processes as well as being flexible and patient when faced with obstacles associated with the changing trial models and increased technology.
ACRP’s Partners in Workforce Advancement5 — whose members include representation from sponsors, CROs, investigator sites, academic institutions, regulatory agencies, and more — has begun working to address these challenges. Partners in Workforce Advancement is a collaborative initiative to:
- grow a more diverse and sustainable clinical research workforce
- set and support standards for workforce competence
- develop and validate the competencies of the workforce
To date, ACRP’s Partners in Workforce Advancement has developed a variety of competency guidelines to guide the development and assessment of competencies across a range of roles. But the work has just begun. Armed with a greater understanding of the roles and competencies needed to be successful working in a highly complex, remote, virtual, and technology-enabled world, we can continue to advance these initiatives. We welcome your participation so that collectively we can all have a positive impact on workforce development and advancement before it’s too late.
References:
- ACRP Special Report: An Assessment of the Adequacy of the Clinical Research Workforce. Available for downloading at: https://acrpnet.org/special-report-an-assessment-of-the-adequacy-of-the-clinical-research-workforce/
- Tufts Center for the Study of Drug Development White Paper. Drug Development Workforce in the Age of Digital Transformation. https://static1.squarespace.com/static/5a9eb0c8e2ccd1158288d8dc/t/
5f033fe4dc3ce86c6b03aa98/1594048485996/White_Paper_FINAL_062920%5B1%5D.pdf - Harper, B. and W. Tate: Teaching Old Dogs New Tricks: Can Competency With Clinical Research Technologies Be Enhanced? https://www.clinicalleader.com/doc/teaching-old-dogs-new-tricks-can-competency-with-clinical-research-technologies-be-enhanced-0001
- ACRP White Paper: The Impact of Increased Technology Use on the Clinical Research Workforce. Available for downloading at: https://acrpnet.org/the-impact-of-increased-technology-use-on-the-clinical-research-workforce/
- https://acrpnet.org/acrp-partners-in-workforce-advancement/
About The Author:
 Beth Harper is the president of Clinical Performance Partners, Inc., a clinical research consulting firm specializing in enrollment and site performance management. Harper also is the workforce innovation officer for the Association of Clinical Research Professionals (ACRP). She has passionately pursued solutions for optimizing clinical trials and educating clinical research professionals for over three decades. Harper is an adjunct assistant professor at George Washington University who has published and presented extensively in the areas of protocol optimization, study feasibility, site selection, patient recruitment, and sponsor-site relationship management. She serves on the CISCRP Advisory Board and the Clinical Leader Editorial Advisory Board, among other industry volunteer activities. Harper received her B.S. in occupational therapy from the University of Wisconsin and an M.B.A. from the University of Texas. She can be reached at 817-946-4728, bharper@clinicalperformancepartners.com, or bharper@acrpnet.org.
Beth Harper is the president of Clinical Performance Partners, Inc., a clinical research consulting firm specializing in enrollment and site performance management. Harper also is the workforce innovation officer for the Association of Clinical Research Professionals (ACRP). She has passionately pursued solutions for optimizing clinical trials and educating clinical research professionals for over three decades. Harper is an adjunct assistant professor at George Washington University who has published and presented extensively in the areas of protocol optimization, study feasibility, site selection, patient recruitment, and sponsor-site relationship management. She serves on the CISCRP Advisory Board and the Clinical Leader Editorial Advisory Board, among other industry volunteer activities. Harper received her B.S. in occupational therapy from the University of Wisconsin and an M.B.A. from the University of Texas. She can be reached at 817-946-4728, bharper@clinicalperformancepartners.com, or bharper@acrpnet.org.

