Caregiver Distress: The Hidden Burden Of Dementia With Lewy Bodies — And Why Measuring It Matters
By Anthony Caggiano, MD, Ph.D., and Theresa Devins, DrPH, MS, Cognition Therapeutics

Dementia with Lewy bodies (DLB) is one of the most difficult neurodegenerative diseases to manage for patients and their caregivers. While the clinical focus is appropriately centered on the patient, caregivers experience many challenges, including distress — the emotional, psychological, and physical strain of providing daily care — which often remains hidden. Caregivers’ levels of distress must be evaluated to better understand the impact of DLB on patients, families, and society overall.1
“As we look at ways to get a full assessment of experimental treatments and whether they are providing truly meaningful results, it’s vital to consider the caregiver experience, in addition to the patient experience,” said James E. Galvin, MD, MPH, director of the Comprehensive Center for Brain Health at the University of Miami Miller School of Medicine. “For diseases like DLB, the caregiver is already an essential partner with the clinician, so formalizing their involvement and measuring their lived experience could be valuable.”
What DLB Caregivers Experience
Caregivers of patients with Alzheimer’s disease (AD) often experience and report high levels of distress as they attempt to manage cognitive, functional, and behavioral symptoms of the person they are caring for. The caregiver’s perspective provides more of an “outside-in” view on disease progression and its broader impact. DLB, a related neurodegenerative condition, has been reported to be even more burdensome and stressful for caregivers, because of its fluctuating symptoms with severe swings in cognition and attention, behavioral symptoms like hallucinations, delusions, and anxiety, as well as motor issues (like falling) that require constant vigilance.2,3 And unlike AD patients, DLB patients can be aware of their declining cognitive and functional abilities, triggering greater anxiety and depression.
An emerging understanding is that the caregiver’s role is more than that of a passive bystander; it’s as an integrated team member caring for the patient. We must understand how the patient’s behavior and disease manifestations impact the world around them, including their caregivers, to deliver better patient care.
How Caregivers Fill Knowledge Gaps
Patients’ self-reported observations of their condition and even assessments by medical professionals don’t always capture the scope of a patient’s DLB experience. In fact, when it comes to clinical trials, many experts agree that caregiver-reported outcomes (CROs) are as important as traditional patient-reported outcomes (PROs) because they may reflect changes that otherwise go undetected during clinical visits. Principal investigators, study staff, and other healthcare providers only report data as they observe it during study visits, which are inherently a small sample of time with the patient. One caregiver shared that her husband would sometimes fail to recognize the bathroom, occassionally urinating in the corner of their bedroom. Yet on other days, he had no trouble identifying and using the bathroom appropriately.
For many caregivers, one of the greatest challenges is what’s known as “showtiming” — when patients appear much sharper and more engaged during those doctor visits than they are at home. DLB patients often have frequent “ups and downs” referred to as the “wobble” by many treating physicians. This can make it difficult for physicians - or even family or friends who only see the patient in brief, infrequent visits - to appreciate the full scope of the disease and frequent symptom fluctuations that caregivers observe, often leaving them feeling frustrated and unsupported in their role.
How Caregiver Input Enriches Trial Data
In the SHIMMER Phase 2 study of zervimesine (CT1812) in DLB, participants treated with zervimesine showed robust improvements across four major symptom domains: behavior, function, cognition, and movement. Notably, zervimesine demonstrated an 86% improvement in behavioral symptoms, as measured by a scale called the Neuropsychiatric Inventory (NPI)4, compared to placebo. Importantly, this improvement in patient symptoms was accompanied by a notable reduction in caregiver distress, with caregivers of zervimesine-treated patients reporting significantly less stress related to managing these challenging behaviors. The NPI scale was developed nearly 30 years ago to allow clinicians to systematically score a wider range of behaviors than standard tests used in neuropsychiatry at that time. The intent was to capture behavior changes that patients that may be unable to recognize or recall themselves. The self-administered test allows the caregiver to report behavior and mood changes across standard cognitive domains.
The NPI scale is scored by severity of specific symptoms and is a rating of the caregiver’s distress due to behaviors exhibited by the person they are caring for. Examples include the emotional toll of responding to unpredictable, confusing, and sometimes alarming episodes such as when patients experience hallucinations, hear voices, or have outbursts that may be aggressive or include hurtful statements. To our knowledge, this is the first instance of a drug’s effects being meaningfully linked to outcomes reported by both patients and their caregivers.
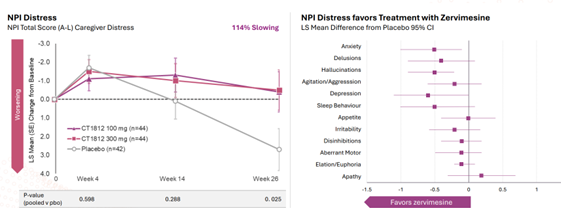
Figure 1. NPI results showing Reduction in Caregiver Distress
As new treatments for DLB — and age-related neurodegenerative diseases more broadly — advance toward late-stage trials and potential approval, including measures like the NPI Caregiver Distress scale could help capture the full impact of treatment on both patients and families.
Why Caregiver Distress Needs To Be Measured Now
Caregiver distress is often the tipping point that leads to institutionalization of the person they are caring for. Clinicians report that people with DLB are often placed in care facilities not because of memory loss, but because caregivers — often aging spouses or adult children — feel overwhelmed and can no longer manage their loved one’s severe behavioral or motor symptoms on their own. A therapy that reduces caregiver distress in addition to improving behavioral symptoms and patient daily functioning could allow DLB patients to stay at home longer, preserving family bonds and reducing overall healthcare costs.
The caregiver voice must be carefully planned into the design of each study, particularly for indications known to cause caregiver distress. Caregivers are crucial to building programs that attempt to capture the totality of the disease. At Cognition Therapeutics, we are committed to listening to their historically unheard voices and amplifying their perspectives in future studies.
References:
- Holden SK, Bedenfield N, Taylor AS, et al. Research Priorities of Individuals and Caregivers With Lewy Body Dementia: A Web-based Survey. Alzheimer Dis Assoc Disord. 2023;37(1):50-58.
- Yuuki S, Hashimoto M, Koyama A, et al. Comparison of caregiver burden between dementia with Lewy bodies and Alzheimer's disease. Psychogeriatrics.
- Tahami Monfared AA, Meier G, Perry R, Joe D. Burden of Disease and Current Management of Dementia with Lewy Bodies: A Literature Review. Neurol Ther. 2019;8(2):289-305.
- Cummings J. The Neuropsychiatric Inventory: Development and Applications. J Geriatr Psychiatry Neurol. 2020 Mar;33(2):73-84.
About The Experts:
 Anthony O. Caggiano, MD, PhD is chief medical officer and head of R&D at Cognition Therapeutics, where he directs clinical development of zervimesine (CT1812) and oversees the company’s preclinical research on sigma-2 receptor modulators. He joined Cognition from Neurotrauma Sciences, where he served as CMO and R&D head for stroke and TBI programs. Previously, he spent 17 years at Acorda Therapeutics, rising to SVP of R&D, where he led development for multiple neurological indications and built integrated internal and external research programs. Earlier, he held executive roles at Constant Pharmaceuticals and Aeromics. Dr. Caggiano earned both his MD and Ph.D. from the University of Chicago and holds a B.A. from the University of Virginia.
Anthony O. Caggiano, MD, PhD is chief medical officer and head of R&D at Cognition Therapeutics, where he directs clinical development of zervimesine (CT1812) and oversees the company’s preclinical research on sigma-2 receptor modulators. He joined Cognition from Neurotrauma Sciences, where he served as CMO and R&D head for stroke and TBI programs. Previously, he spent 17 years at Acorda Therapeutics, rising to SVP of R&D, where he led development for multiple neurological indications and built integrated internal and external research programs. Earlier, he held executive roles at Constant Pharmaceuticals and Aeromics. Dr. Caggiano earned both his MD and Ph.D. from the University of Chicago and holds a B.A. from the University of Virginia.
 Theresa R. Devins, DrPH, MS is vice president of clinical operations at Cognition Therapeutics, where she oversees all ongoing and planned clinical trials for the company’s lead candidate, zervimesine (CT1812). Dr. Devins has more than 25 years of global clinical research experience, including leadership roles in complex neurological trials. At Cognition, Dr. Devins managed enrollment for the SHINE study in mild-to-moderate Alzheimer’s disease and the SHIMMER study in dementia with Lewy bodies, and helped initiate the Phase 2 MAGNIFY and START studies. Previously, she was director of clinical operations at Eisai, leading Alzheimer’s and sleep disorder studies while advancing diversity in clinical trial recruitment through community partnerships. Earlier in her career, she held roles at Regeneron and Boehringer Ingelheim.
Theresa R. Devins, DrPH, MS is vice president of clinical operations at Cognition Therapeutics, where she oversees all ongoing and planned clinical trials for the company’s lead candidate, zervimesine (CT1812). Dr. Devins has more than 25 years of global clinical research experience, including leadership roles in complex neurological trials. At Cognition, Dr. Devins managed enrollment for the SHINE study in mild-to-moderate Alzheimer’s disease and the SHIMMER study in dementia with Lewy bodies, and helped initiate the Phase 2 MAGNIFY and START studies. Previously, she was director of clinical operations at Eisai, leading Alzheimer’s and sleep disorder studies while advancing diversity in clinical trial recruitment through community partnerships. Earlier in her career, she held roles at Regeneron and Boehringer Ingelheim.
