Clinical Operations Cost Variation: Oncology vs. Non–Oncology

By Mathini Ilancheran, Senior Research Analyst, Clinical Research, Beroe Inc.
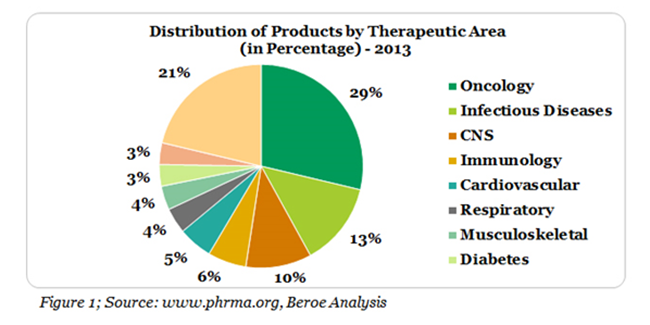
The number of products in pipeline within Oncology therapeutic area was 3436 in 2013, which contributes to more than 29% of the total R&D projects (Figure 1).1 Cancer related cases are also estimated to rise up to 22 million within the next two years.2 Keeping these statistics in mind, pharmaceutical companies have earmarked a major proportion of their R&D investment on cancer related research.
With more oncology clinical trials in the pipeline of most large pharma, there is question of why the operating cost for Oncology trials are higher than that of other therapeutic areas. Let us take a detailed look into this question.
How are Oncology trials different?
Oncology trials differ from other therapeutic areas based on three main parameters: Patient recruitment, Clinical trials cost and Clinical Research Associate (Clinical Monitor) skill set.
Patient Recruitment: Phase I trials for each therapeutic area involves healthy volunteer studies. However, since oncology studies more often have toxic side effects, early phase trials for oncology would recruit patients with advanced, heavily treated or untreatable disease condition.3 This in turn adds on to a higher patient recruitment cost since finding the right patient cohort with the appropriate cancer subtype for the treatment is difficult.
Oncology patient enrolment between the First Subject Initiated to Last Subject Randomized (FSI – LSR), is the highest relative to the number of studies at 106.3 weeks through phase II – IV studies (Figure 2).
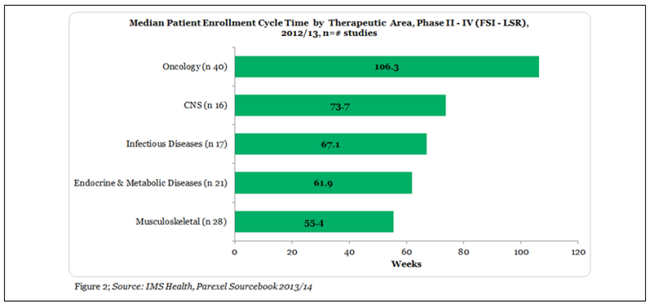
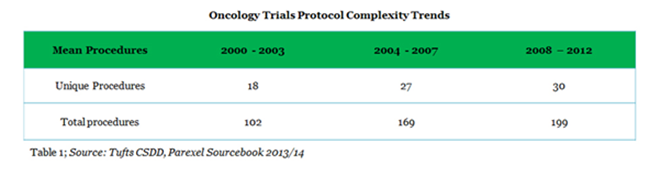
There are many reasons for longer cycle times of Oncology trials mainly in phase II and III trial stages. The most significant of them include protocol complexity (Table 1), longer treatments, higher number of procedures and biomarker search. Issues of multiple diseases, multiple end points, and the increasing number of subjects also play its part.4
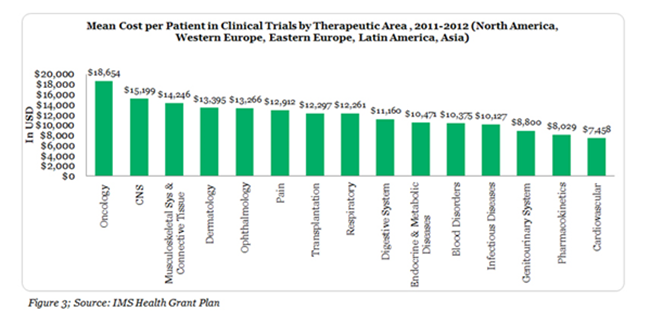
Clinical Trial Cost: Oncology clinical trial monitoring needs to be more intensive compared to other areas due to the severity of the disease mainly in early stage of the trials. This factor combined with the regulatory demands adds on to the higher cost of Oncology clinical trial conduct.3 The total cost negotiated with an investigator to complete all protocol activities for a single patient is highest for oncology trials, increasing the costs even further. This cost includes procedures, personnel costs, travel reimbursement and overhead excluding conditional procedures and site costs (Figure 3).
Clinical Research Associate/ Clinical Monitors: Recruiting the right CRA for Oncology clinical monitoring remains a challenge. Though the basic skills for CRA are similar for all therapeutic areas, the complexity of the disease requires specific skills and expertise for respective type and subtype of cancer being studied.3 The specialized skill set adds on to the FTE rate of an Oncology CRA being on a higher side compared to other therapeutic areas. In the Oncology space, CRAs are given fewer protocols because it increases quality and allows them to be experts in their therapeutic area. This increases the need for higher number of CRA’s to manage all the protocols.
Case Study: CRA Cost Comparison – Oncology vs. Non-Oncology
Oncology is an area where there is a need for specialized skill sets as said before; hence attracts a higher remuneration. Increase in number of clinical studies focusing on oncology every year is driving the need for more CRAs with therapeutic area expertise in oncology. This is a true example of supply outstripping demand and driving the price higher.
Figure 4 below shows an example of percentage change in the FTE rate of an Oncology vs. Non-Oncology CRA in Japan at three different experience levels.
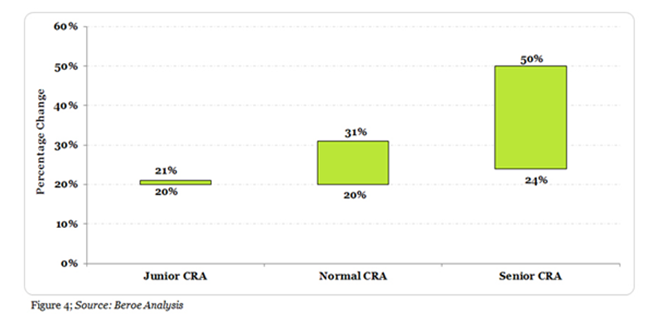
The minimum and maximum monthly FTE rate for Jr. CRA with 1 – 3 years of oncology expertise is 20 – 21% greater than that of a non-oncology expert. Similarly, for a CRA with 3 – 5 years of expertise, the minimum to maximum monthly rate is 20 - 31% greater than that of a non-oncology CRA. Finally, the Sr. CRA rates with greater than 5 + years’ experience is 25-50 % higher than that of a non-oncology expert.
References:
- http://www.phrma.org/sites/default/files/pdf/2013innovationinthebiopharmaceuticalpipeline-analysisgroupfinal.pdf
- http://www.rootsanalysis.com/insights/view_document/pharma-landscape-mapping-the-research-focus/65.html
- https://vertassets.blob.core.windows.net/download/4e609a4c/4e609a4c-3502-49dc-9764-d343a5c8d387/14_15_c.pdf
- http://www.clinicalleader.com/doc/oncology-trials-facing-longer-cycle-times-report-finds-0001
