2021 Clinical Research Site Survey Findings: A Year In Flux
By Jimmy Bechtel, Society for Clinical Research Sites
 Since its inception in 2012, the Society for Clinical Research Sites (SCRS) has conducted an annual survey of clinical research site community members to derive insights, reveal trends, and ensure the site perspective is represented within the broader industry. In the 2021 Site Landscape Survey, more than 500 individuals across a spectrum of roles and organizations answered questions regarding the current state of their operations, partnerships, finances, and experiences with technology. The results echo patterns identified in past surveys, but they also reflect the impact of the ongoing global pandemic, as well as the influence of newer trends driving rapid transformation in clinical research.
Since its inception in 2012, the Society for Clinical Research Sites (SCRS) has conducted an annual survey of clinical research site community members to derive insights, reveal trends, and ensure the site perspective is represented within the broader industry. In the 2021 Site Landscape Survey, more than 500 individuals across a spectrum of roles and organizations answered questions regarding the current state of their operations, partnerships, finances, and experiences with technology. The results echo patterns identified in past surveys, but they also reflect the impact of the ongoing global pandemic, as well as the influence of newer trends driving rapid transformation in clinical research.
For example, the data indicates how the increasing digitalization and globalization of medical research affects clinical research sites, with some sites realizing increased revenue and profits while others saw study opportunities and profits decline. Similarly, trends explored in past research, such as the adoption of eClinical technologies, continue to shape operational strategies.
To understand the full impact of these factors on sites, and the associated challenges and opportunities, the 2021 survey results are categorized into four themes: Relationship dynamics between sites and sponsors or clinical research organizations (CROs), the impact of the coronavirus pandemic, sites’ financial health, and the impact of new study design models like decentralized clinical trials (DCTs).
Site Success & Sustainability
The survey kicked off with questions to establish a baseline of the top factors that determine site success and sustainability. Sites reported that recruiting patients, identifying study opportunities, and improving operational efficiency are the three most important performance objectives to be successful. Most sites regularly measure their performance against these objectives by keeping track of the number of studies awarded, screening and enrollment metrics, and total revenue.
Uncertainty And Opportunity In The COVID-19 Pandemic
Building on a trend that emerged in the 2020 Site Landscape Survey, sponsors and CROs continue to implement technologies and study conduct methodologies that facilitate fully decentralized or hybrid trials, enabling research activities such as patient site visits to take place away from centralized sites when appropriate. Two-thirds of sites were approached by sponsors or CROs about conducting new clinical trials using a hybrid model, whereas 37% were approached about conducting fully decentralized clinical trials. In each case, sites overwhelmingly chose to participate, citing previous experience, the benefits to participant populations, and opportunities to modernize as the primary considerations in those decisions. Of the sites that chose to not participate in trials of either model, respondents expressed concerns about recruiting, lack of familiarity with the trial model, financial concerns, in addition to safety, privacy, or regulatory issues as the reasons they declined to participate.
Technology Impacting Sites
The data suggest that despite the challenges of safety precautions or movement restrictions imposed by pandemic mitigation measures, sponsors continue to design protocols that favor hybrid or traditional models as opposed to a fully decentralized trial design, underscoring the importance of physical research sites. As a case in point, members reported that in 2020 and 2021, 80% of trials had been conducted using a traditional model of conduct.
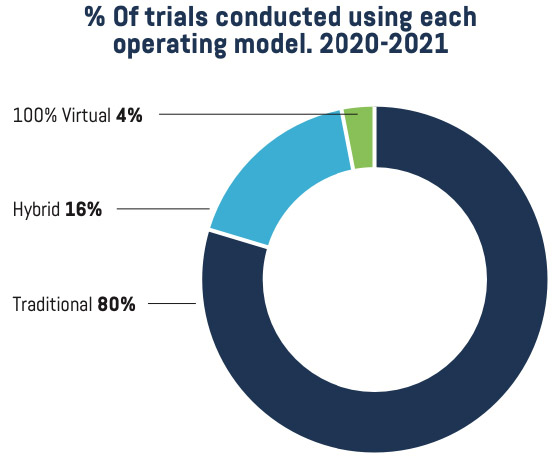
The 2021 Site Landscape survey data suggests that more sites are prepared for, experienced in, and willing to participate in hybrid trials versus fully decentralized clinical trials. As mentioned previously, 37% of sites were approached about conducting new trials using fully decentralized models, and of those, three-quarters chose to participate based on experience and preparedness. The sites that did not participate cited lack of familiarity with the trial model and inadequate budget. Interestingly, only a third of members were approached about converting active trials to a full DCT model.
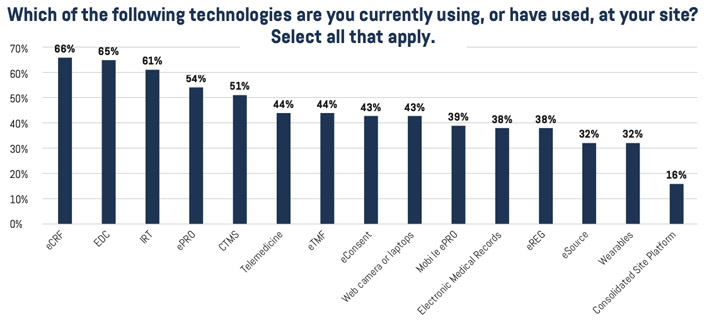
When it comes to the technology infrastructure that enables remote clinical trial conduct, sites prefer to use solutions provided by the sponsor or CRO, or a combination of their own and sponsors’. Put simply, sites do not want to bear full responsibility for investing in and providing technology for DCT or hybrid trials. Most sites use some technology and are piloting or planning to invest in new technology. Solutions with which they are familiar include eCRF, EDC, IRT, ePRO, CTMS, telemedicine, eConsent, and eSource software. The primary factors impeding adoption are cost and concerns revolving around the support for sites and patients.
When asked whether they feel that sponsors and CROs are compensating sites fairly for the work and effort involved in conducting decentralized trials, members were divided. Forty-two percent said in some or all instances they felt they were fairly compensated, while 48% disagreed. They noted that training takes longer and costs for infrastructure and administration are higher. Based on the data presented earlier, study budgets have increased modestly, if at all. Higher costs coupled with stagnant study budgets resulted in less revenue and profit. As the number of sites conducting DCTs grows, sites can share more insight with sponsors and CROs about the time needed to manage these types of trials, and budgets should be adjusted accordingly.
Looking Ahead
Sponsors, CROs, and clinical research sites share the common goal of conducting successful studies to bring new therapeutic solutions to patients in need. It is clear from the results of this survey that priorities are aligned well in some areas – such as the benefits of global, tech-enabled clinical trials – but there is still room for improvement in others, including communication and contract negotiations.
Download the full report here, available to SCRS members and Global Impact Partners. SCRS collects new data throughout the year and shares insights at each SCRS Site Solutions Summit.
About The Author:
 Jimmy Bechtel is vice president of site engagement for Society for Clinical Research Sites (SCRS). He is in charge of developing and executing the company's site-facing initiatives and works closely with key industry partners to build out various SCRS partnership programs. Bechtel brings experience from both the site and sponsor sides of the clinical research industry. In his site experience, he served as a data specialist, patient recruiter, and operations manager. In his pharma experience, he worked in innovation project management, encouraging a site-centric environment and creating ease for sites working with a major sponsor company.
Jimmy Bechtel is vice president of site engagement for Society for Clinical Research Sites (SCRS). He is in charge of developing and executing the company's site-facing initiatives and works closely with key industry partners to build out various SCRS partnership programs. Bechtel brings experience from both the site and sponsor sides of the clinical research industry. In his site experience, he served as a data specialist, patient recruiter, and operations manager. In his pharma experience, he worked in innovation project management, encouraging a site-centric environment and creating ease for sites working with a major sponsor company.
