Clinical Trial Utilizes DoseSmart Medication Adherence Platform And Smart Pill Bottle
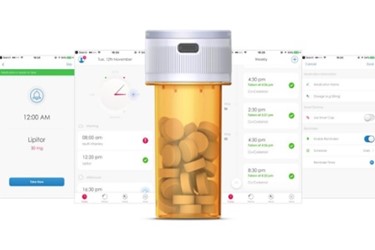
San Francisco, CA (PRWEB) - DoseSmart Inc. announced today that their proprietary medication adherence technology is currently being used in a new clinical trial (clinicaltrials.gov ID NCT03460587) which focuses on helping stroke survivors comply to their medication regime. The clinical trial, "Long-term Telerehabilitation for Patients with Stroke," is being conducted by Dr. Steven C. Cramer, Professor of Neurology, Anatomy & Neurobiology as well as Physical Medicine & Rehabilitation at the University of California, Irvine. The study will examine new ways to deliver stroke rehabilitation and preventative care in the home of stroke survivors. DoseSmart’s patented and portable SmartBottle stores, dispenses and tracks prescription medication in conjunction with a companion application and is designed for both patient adoption and care team support. The trial will utilize the SmartBottle to record a daily dose of study-administered medication in simple format for efficient reporting to researchers in real time. DoseSmart’s customizable companion application reports directly from the SmartBottle to the clinician researcher without the need for the patient involvement, significantly decreasing human error and improving the clarity of data and the quality of reporting.
“DoseSmart developed a custom version of their software that adapted to our study needs and provides us with real time adherence data on each study medication dose. In addition, the SmartBottle fits on the industry standard One-Click bottle created by Centor, seamlessly integrating the technologies with our patients’ regimens.” said Dr. Cramer. “We believe that DoseSmart will not only yield more accurate data for the purposes of our study, but potentially represent a tremendously useful tool for a broad scope of patient medication adherence.”
“We are proud to be included in such an important and prestigious study such as Dr. Cramer’s,” stated DoseSmart spokesman David MacVittie. “We are confident that the study will bring to light the importance of regimented medication adherence as well as provide patients with an effective and easy-to-use system that yields optimal results from medications.”
The trial commenced on July 1, 2018 with an estimated study completion date of December 31, 2018.
About DoseSmart:
DoseSmart is the revolutionary way to ensure patients remember to take their pills on time, every time. DoseSmart Inc.’s patented technology provides a simple three-part solution to a complex problem of medication non-adherence. The first part is an easy-to-use Smartphone application where patients create custom schedules and set reminders for time, day and dosage of medications. The second part is the SmartBottle which stores, dispenses and tracks prescription medication and sends adherence data to the backend. The third part is a web-server driven data storage, presentation and analytics solution for use by care team support and/or clinical trials and research organizations. http://www.mydosesmart.com/
About Dr. Steven C. Cramer:
Dr. Steven C. Cramer is a Professor of Neurology, Anatomy & Neurobiology, and Physical Medicine & Rehabilitation at the University of California, Irvine. He is also the Associate Director of the Institute for Clinical & Translational Science at UC Irvine, and co-PI of the NIH StrokeNet clinical trials network. Dr. Cramer’s research and clinical trials focus on neural repair after central nervous system injury in humans, with an emphasis on stroke and recovery of movement.
Study info:
http://www.clinicaltrials.gov/ct2/show/NCT03460587
http://www.mydosesmart.com
Source: PRWeb
View original release here: https://www.prweb.com/releases/clinical_trial_utilizes_dosesmart_medication_adherence_platform_and_smart_pill_bottle_7_23_18/prweb15631426.htm
