Collaborating With Patients To Design More Effective Hybrid Trials
By Life Science Connect Editorial Staff

At the center of every clinical trial is one goal: to bring an innovative, effective therapy to a population in need of treatment. What may seem intuitive in theory – actively listening to patients and caregivers about their concerns – has not always been used in practice. In many cases, righting the ship toward more patient-centric trial design may be met with resistance, but at Sanofi Digital Technologies, care teams are collaborating with critical stakeholders to design trials that prioritize patients. Their holistic approach to patient centricity is a testimony to how incorporating frontline perspectives and digital technologies will improve overall experience and reduce the clinical trial burden.
What Is Patient-Informed Development?
Designing successful, patient-centric clinical trials necessitates information gathered via collaboration with crucial stakeholders. Sanofi defines patient centricity as “the commitment to listen and translate patient insights into actions that develop new healthcare solutions with meaningful outcomes, address unmet needs, and enhance health-related quality of life.”
Interactions begin as early as the conceptual discovery phase and proceed through research and development (R&D) into post-approval activities (Figure 1). In the earliest phases, this entails meeting with different advocacy organizations and individuals living with specific diseases to determine what they are looking for in their ideal healthcare solutions. Using the results of these discussions, discovery teams will strategize at the bench to propose new pathways to addressing unmet needs. During the development stages, this data helps determine how to define health value if a product enters the commercial and approval process. Amid these considerations, Sanofi is also looking closely at how best to collect data for clinical trial outcomes and whether digital technology is the most accessible route. Throughout the process, consistent engagement ensures that their approaches and the goals of the community are completely aligned.
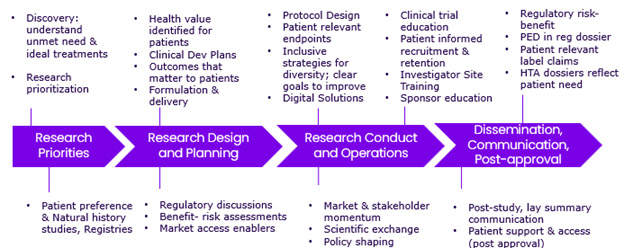
Figure 1. Examples of how insights are incorporated into each phase
How Should A Company Tackle Patient-Informed Development?
Building more patient-centric trials may appear daunting from the outset. However, there is significant shared value in developing clinical trials that serve as healthcare opportunities and integrate into patient lives easily. At Sanofi, this began at a grassroots level and transformed into a company-wide restructuring. In the years since, it has been implemented to accommodate a bevy of systems, including R&D, regulatory policy, and public and corporate affairs, with yearly objectives to verify that workflows are effective. Opting to embed patient data across different teams has led to more efficiency, momentum, and improved results. Per Vicky DiBiaso, global head of patient informed development and health value translation at Sanofi: “When everyone rallies around it, you have a groundswell.”
Presently, 100% of Sanofi’s clinical development programs, disease indications, trials, and studies are informed by patient perspectives. All studies have remote and digital capabilities to reduce barriers to access and, as a result, increase diversity and inclusion. The company engages directly with individuals in the patient community as well as healthcare providers and advocacy leaders. Currently, they are working with stakeholders in 33 different countries and supported by 130 advocacy organizations to reach their objectives. Advocacy organizations provide insight into what forms of life cycle management are preferred, including what effectively recruits and retains patients and what questions they and their loved ones need answered to make informed decisions about their options.
Sanofi conducts patient preference studies to explore what patients are looking for in trials and confirm or invalidate hypotheses. They also draw upon clinical trial experiences, using clinical outcomes assessments (COAs) and health data. Participants provide data through digital technology, including wearable devices and smartphones, but further data is retrieved via electronic health records, social media listening, and literature review. These expansive data gathering mechanisms are used to implement a comprehensive strategy for change and to reduce the clinical trial burden as much as possible.
What Are The Emergent Trends?
Currently, the clinical trial space is increasingly leveraging digital and remote technologies to offer decentralized clinical trials (DCTs) with greater accessibility. Digital and artificial intelligence (AI) technologies offer the opportunity to accelerate development, reduce costs, and improve outcomes. Presently at Sanofi, a key performance indicator (KPI) is ensuring that 100% of Phase 2 and 3 studies have remote capabilities.
Digital biomarkers are an exciting prospect in the clinical trial space. Much like traditional biomarkers, they provide insight into an individual’s biological data, but said data is retrieved with digital devices like smartphones or wearable devices. They are used throughout clinical development and can replace or supplement traditional biomarkers and clinical outcome assessments.
When it comes to using electronic patient-reported outcomes (ePRO), high usability and accessibility are critical. ePRO allows trial participants to provide direct feedback on their health via an electronic device like a phone or tablet. 1 It eliminates the need for paper forms and allows trial data to be tracked rapidly and efficiently. During early adoptions, Sanofi recommends working with patients and advisors to conduct walkthroughs, ensuring that patients can see and input information into handheld devices. When conducting ePRO, users need access to support to troubleshoot any questions they have. The goal is to offer a one-stop-shop experience rather than forcing users to navigate multiple applications and devices at a time.
Overall, flexibility continues to be the most vital element of clinical trial access; people need an array of options and support. In the process of their studies, Sanofi found that some clinical trial participants prefer the ability to visit the clinic to see their physicians. In this case, flexible models can help ensure patient satisfaction.
A New Culture Of Clinical Trials
Building patient-focused clinical trials yields ample benefits to drug sponsors and patients in need of effective treatments. Comprehensive integration of patient data from the discovery phase through to commercial manufacturing is an effective mechanism toward that end. Patient experience teams can enhance their touchpoints by way of partnerships with advocacy organizations and digital technologies. Ultimately, these cultural shifts stand to increase accessibility and diversity for clinical trials while helping to bring more effective therapies to patients.
The above content originally appeared on the Clinical Leader Live podcast; you can watch the full episode here.
References
- Ryerson, N. What is EPRO in clinical trials? Antidote. https://www.antidote.me/blog/what-is-epro-in-clinical-trials
