CRO Oversight Essentials
By Sandra "SAM" Sather and Jennifer Lawyer, Sandra "SAM" Sather and Jennifer Lawyer, Clinical Pathways, LLC
 Since clinical trials have become increasingly complex with more data being collected, this consequently puts more pressure on sponsors to improve efficiencies while still maintaining quality.
Since clinical trials have become increasingly complex with more data being collected, this consequently puts more pressure on sponsors to improve efficiencies while still maintaining quality.
Although CROs are commonly entrusted to handle the bulk of the clinical trial process, it’s still the sponsor’s responsibility to ensure the quality of that work. As such, sponsors need to proactively identify and manage the risks involved with working with a CRO. This kind of oversight can help reduce the likelihood of negative consequences that could impact the quality of a trial.
Where Responsibility Of Oversight Lies
In the 2013 FDA guidance on risk-based monitoring, the section “Delegation of Monitoring Responsibilities to a CRO” (section VI C) clarifies that the sponsor can delegate monitoring to a CRO, but the sponsor retains the responsibility to oversee the work of the CRO. Likewise, ICH E6(R2) (section 5.2) allows for the transfer of any or all trial-related duties to a CRO, with the reminder that the sponsor is ultimately responsible for the quality and integrity of the clinical trial. The recently updated (September 2021) FDA Bioresearch Monitoring (BIMO) Program Compliance Program Guidance Manual (CPGM) for sponsors includes a new section, III. F. “Outsourced Services,” related to the oversight of CROs and other vendors as inspectable items. The agency wants to know how the sponsor ensures that its outsourced activities comply with FDA regulations, the protocol, and GCP. Additionally, in many other parts of the updated CPGM, there are new items that focus on vendor selection and oversight (e.g., an expanded section, N. “Data Collection and Handling”). In short, the sponsor can transfer duties, but not the responsibility of proper oversight of the clinical trial.
Oversight does not mean micromanagement. A CRO is contracted for its specific capabilities. It is important to allow the CRO to do whatever it does best and not to overcontrol how they implement. Completing a task in a different manner does not necessarily mean it is wrong. If CROs can use their expertise, it may improve outcomes, which is the goal of outsourcing. A good oversight plan includes establishing clear roles and responsibilities.
When a sponsor has a positive experience with a CRO, they should be cautious in expanding the CRO’s services to different types of clinical trials, such as a different therapeutic area, new technologies, or from Phase 1 to Phase 2 trials. CROs want to expand their expertise with willing sponsors. Just because they did a good job in one area does not guarantee the same performance in new or expanded areas. CROs may lack experience or training and may have less mature systems or processes than are needed for a successful outcome. Sponsors that do change or expand a CRO’s scope may be provided various incentives to do so, but the resource strain for the sponsor’s study team can be huge, with many expensive negative consequences. The qualification and planning for oversight of the CRO have to be adjusted. If the sponsor does not have experience in the assessment and management of expanding a CRO’s scope, the risk assessment should look at the critical processes’ impact from a CRO and sponsor perspective. There are many proactive factors that could be agreed upon prior to the written agreement to better support the sponsor study team’s efforts and to ensure quality.
A good communication plan for all involved supports the efforts of the CRO while keeping the sponsor informed on the progress, or it can provide an early signal of a potential risk. The sponsor supporting the CRO is a critical step, since proper support leads to efficiency, high quality output, less rework, and less need for micromanagement. Maintaining an adaptive approach to the oversight process is essential for a growing sponsor.
Successful CRO Oversight Relationship-Building Essentials
The key to a successful partnership with the vendor is an effective two-way supportive relationship. All parties involved are vested in the success of the clinical trial. It is important to remember this common goal, especially when conflict may occur. The following are some practical tips to build the relationship:
Sponsor/Vendor Teams Should:
- clearly define critical processes and associated roles, responsibilities, and expectations
- know and understand agreements, scope, reporting obligations, and quality expectations
- agree on a team communication plan, including an escalation plan
- establish positive working relationships through open and honest communication
- communicate concerns, issues, and changes early to avoid surprises.
Sponsors Should:
- form long-term partnerships with vendors.
- establish a solid vendor selection process and criteria that involve the teams that will be overseeing the critical processes that are to be transferred.
- assess if the vendor has adequate resources with qualified individuals and will follow GCP, the protocol, processes, and applicable regulations.
- plan for succession management.
- maximize consistency of practices.
- separate operations from budget management, but ensure that consultation occurs.
- stay involved with projects and be available when decisions are needed for critical processes.
- provide realistic assessment of timelines and needs.
- remain flexible.
- listen, clarify, learn, and show respect.
- focus on outcomes and not process.
Also, Sponsors Should Remember That CROs Want:
- to be treated with courtesy and respect, with their expertise recognized.
- to work together with the sponsor in a team environment.
- good lines of communication.
- clearly defined priorities and expectations, with shared objectives.
- a credible sponsor who is responsive to their need for information and decision-making.
- to have timely, constructive feedback rather than blame or assumptions.
- sponsors that allow them to use their expertise and experience.
Components Of Effective Sponsor-CRO Oversight
The essential components of CRO oversight should support the CRO in meeting timelines and milestones, maintaining a positive relationship, and safeguarding the quality of the work it provides. Remember, an oversight approach should not solely rely on timelines and milestones, as the relationship could become dysfunctional and would likely lead to poor outcomes that impact other oversight components, like quality. The focus should, instead, be on supporting what the CRO needs to attain the timelines and milestones rather than solely micromanaging the numbers.
The factors that support the sponsor’s ability for a successful oversight strategy are established during negotiations. Input from the clinical operations team should be sought before negotiations are complete. Without proper planning, there could be a lack of adequate resources, talent, and support from the CRO to implement the plan. A supported relationship between the sponsor and vendor drives quality and positive outcomes.
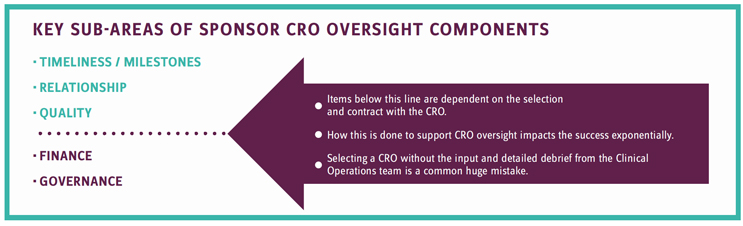
See the following image for key sub-areas of sponsor- CRO oversight components. Note that items below the line are dependent on the selection and written contract with the CRO. How this is done to support CRO oversight impacts the success exponentially.
Regulatory Focus On CRO Oversight
As mentioned, the BIMO CPGM for sponsors was recently updated in September 2021 to include an entire section on outsourced services, which includes CRO oversight. The CPGM is used to guide FDA inspectors when conducting an inspection for subject protection and data quality and integrity as part of the FDA’s BIMO Program. The agency has a greater focus on inspecting outsourced trial-related activities that are critical to the clinical trial or that deviated from the regulations. The FDA wants to know if the sponsor has processes and procedures in place for selection of a CRO, including determining that the CRO will follow GCP and regulations and how they confirm the qualification of CRO personnel. Additionally, the FDA will look for 1) written agreements for roles and responsibilities, 2) SOPs for audits, communication plans, escalation plans, and contingency plans, 3) the overall oversight plan, and 4) protocol-specific training provided to the CRO. A common inspection finding is a failure to ensure proper monitoring of the clinical trial. If the CRO is contracted to provide the direct monitoring of the clinical trial, the sponsor needs to ensure that their monitoring plan and procedures will meet the needs of the particular clinical trial, with continual review and oversight during the conduct of the clinical trial.
Keep in mind that even if the sponsor performed CRO oversight, if it is not written down, it did not happen. Additionally, oversight activities that involve the CRO’s time need to be in writing and agreed upon, or they are at risk of not happening. For example, site-monitoring oversight visits in a sponsor- CRO oversight plan may not occur if not agreed to and in writing. The CRO is likely to push back since the visits may take away from the monitor’s time onsite per the monitoring plan and other billable services. The sponsor should be sure its oversight is clear to the CRO, including how that oversight could interact with clinical trial activities or may impact the CRO and/or the sites. Define the oversight early on in the relationship to prevent delays in contract and scope negotiations and associated out-of-scopes. It is common that milestones can overshadow oversight. Remember, the life of a sponsor monitor is very different than one of a CRO monitor.
Plan For An Evolving Process
When a sponsor transfers responsibilities to a vendor, they retain oversight responsibility of the clinical trial. For a sponsor that is transitioning to an outsourcing model or expanding their outsourcing model, it may take some effort to move from a position of implementation to one that is oversight based and to resist the urge to micromanage the vendor. The transfer of responsibilities is required to be in writing per 21 CFR Part 312.52, but the details of the scope of the transfer is expanded upon in contractual agreements and specifications of scope of work between the parties. Many times, this is not detailed enough to specify how the CRO will perform the trial- related activities, how the CRO will oversee their vendors, or how the sponsor will oversee the CRO. This can cause a difference in understanding where the responsibilities lie, which can lead to ineffective clinical trial management and potential critical issues. The sponsor’s oversight of the CRO may include periodic review of the monitoring reports and vendor performance and should include documented communication regarding monitoring progress and any findings. A clear understanding of both parties’ responsibilities, with an adaptive, scalable approach, and the expectations for the conduct of transferred obligations are essential for a successful clinical trial. A sponsor should plan for growth and a process that evolves with maturity.
About The Authors:
 Sandra “SAM” Sather, MS, BSN, CCRC, CCRA, is an industry-leading consultant whose mission is to promote clinical quality systems for sponsors/CROs and investigators/research institutions. She has over 35 years of clinical experience, with a BS in nursing and an MS in education with a specialization in training and performance improvement. Sather is the VP of Clinical Pathways, a consulting firm with clients in the medical device and pharmaceutical industries located in the Research Triangle Park area in North Carolina. She is dual certified by the Association for Clinical Research Professionals (ACRP) for over 15 years (CCRA and CCRC) and a current ACRP Fellow, which is awarded to individuals who have made substantial contributions to the Association and the industry at large. Learn more at www.clinicalpathwaysresearch.com.
Sandra “SAM” Sather, MS, BSN, CCRC, CCRA, is an industry-leading consultant whose mission is to promote clinical quality systems for sponsors/CROs and investigators/research institutions. She has over 35 years of clinical experience, with a BS in nursing and an MS in education with a specialization in training and performance improvement. Sather is the VP of Clinical Pathways, a consulting firm with clients in the medical device and pharmaceutical industries located in the Research Triangle Park area in North Carolina. She is dual certified by the Association for Clinical Research Professionals (ACRP) for over 15 years (CCRA and CCRC) and a current ACRP Fellow, which is awarded to individuals who have made substantial contributions to the Association and the industry at large. Learn more at www.clinicalpathwaysresearch.com.
 As the operations director at Clinical Pathways, Jennifer Lawyer’s focus is on implementing processes to improve quality and on-time delivery for eLearning development and project management. As an eLearning project manager, she ensures the day-to-day processes run efficiently and products are high-quality and completed on time. Prior to joining Clinical Pathways, Lawyer worked as a clinical research professional and a private duty nurse. She holds a BS in psychology, an AS degree in nursing, and two clinical trials research associate certificates (core competencies and advanced topics). She is a member of the Association of Clinical Research Professionals (ACRP) and is working on her professional certification.
As the operations director at Clinical Pathways, Jennifer Lawyer’s focus is on implementing processes to improve quality and on-time delivery for eLearning development and project management. As an eLearning project manager, she ensures the day-to-day processes run efficiently and products are high-quality and completed on time. Prior to joining Clinical Pathways, Lawyer worked as a clinical research professional and a private duty nurse. She holds a BS in psychology, an AS degree in nursing, and two clinical trials research associate certificates (core competencies and advanced topics). She is a member of the Association of Clinical Research Professionals (ACRP) and is working on her professional certification.
