Data Governance In The Clinical Trial Ecosystem
By Jaime Cook, Vice President, Technical Delivery
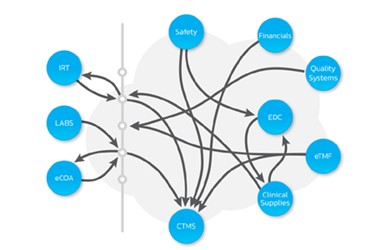
Data are the lifeblood of the drug development process. Expanding volumes of data, multiple data formats and dependence on an increasing number of eClinical systems make data governance essential for the efficient management of data assets across the research and development value chain. Good data governance delivers significant competitive advantage. A high-functioning clinical ecosystem drives better decision-making, operational efficiencies to reduce time and cost, and regulatory compliance to avoid costly errors and rework. This paper discusses the principles of data governance and how they are used to build a business intelligence framework that advances data quality, acquisition, and integration to deliver actionable information for use across the drug development enterprise.
Get unlimited access to:
Enter your credentials below to log in. Not yet a member of Clinical Leader? Subscribe today.
