Decentralized Clinical Trials: Embracing The FDA's 2024 Final Guidance
By Balaji Maddiboyina, Freyr Solutions

In September 2024, the U.S. FDA unveiled a final guidance aimed at advancing decentralized clinical trials (DCTs), through its document Conducting Clinical Trials with Decentralized Elements. This crucial regulatory framework outlines a pathway for integrating decentralized approaches into clinical research, emphasizing patient centricity, accessibility, and technological innovation. For industry, key opinion leaders (KOLs) and healthcare professionals (HCPs), this guidance is not just an update, it is a call to innovate and accelerate the development of new therapies.
What Are DCTs?
Decentralized clinical trials leverage digital health technologies (DHTs) and local healthcare systems to conduct trial activities outside traditional clinical settings. These activities may occur at participants' homes, local clinics, or through telehealth platforms.1,2 DCTs include remote data collection, digital health tools, and local healthcare provider involvement. This model contrasts with traditional site-based trials, offering flexibility through fully decentralized or hybrid approaches based on trial needs and participant preferences.3,4
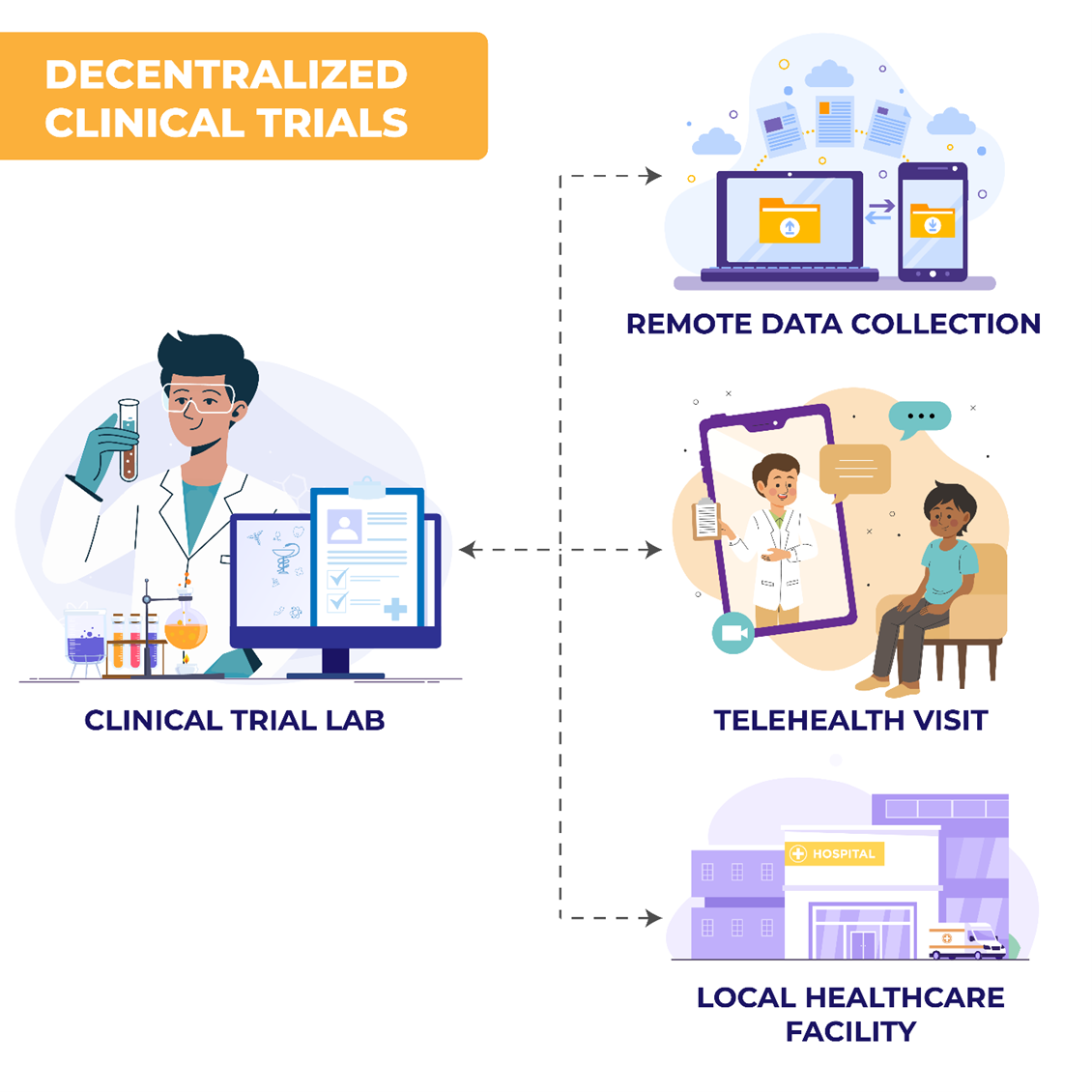
Key Takeaways From The FDA’s Final Guidance
The FDA’s guidance provides several considerations for stakeholders:
- Patient Centricity: DCTs enhance accessibility by reducing participant burden through remote or local trial activities. This model improves recruitment, retention, and engagement, especially among underrepresented populations and those with specific barriers to participation, such as mobility limitations.1,3 By overcoming geographical and logistical barriers, DCTs ensure a diverse participant pool.
- Safety and Data Integrity: DCTs necessitate advanced monitoring systems to ensure accurate data collection across multiple platforms, track adverse events, and prevent medication errors.3,4 The FDA emphasizes adapting safety protocols to each trial's decentralized elements to ensure robust data integrity and participant safety.
- Tailored Flexibility in Trial Design: Sponsors can choose fully decentralized or hybrid designs, based on the complexity of the investigational product and the trial's objectives. Early consultation with FDA review divisions ensures compliance and feasibility of these models.4
- Harnessing the Power of Digital Health Technologies: DHTs are integral to DCTs, enabling real-time data collection and enhancing trial efficiency. Technologies like wearables, mobile apps, and remote monitoring tools enable continuous assessment, improving data precision and accelerating results.3
Key Differences From The May 2023 Draft Guidance
- Clarity on Investigator Responsibilities
Final Guidance: Offers more detailed expectations regarding the delegation of responsibilities to local healthcare providers and third-party vendors. It clarifies that sponsors must ensure that all trial personnel, including remote staff, are appropriately trained and that investigator oversight remains robust, regardless of decentralization.
Draft Version: Lacked specifics on how investigator responsibility should be maintained across decentralized locations.
- Expanded Use of Digital Health Technologies (DHTs)
Final Guidance: Places stronger emphasis on the validation and reliability of DHTs used for data collection. It also encourages sponsors to submit DHT-related protocols early for feedback.
Draft Version: Focused more generally on the potential use of DHTs without detailed direction on their regulatory evaluation.
- Risk-Based Monitoring Framework
Final Guidance: Advocates for a risk-based approach to monitoring, tailored to decentralized elements. This includes the use of centralized and remote monitoring strategies.
Draft Version: Mentioned monitoring but did not emphasize risk-adapted strategies or detail the use of centralized tools.
- Informed Consent Processes
Final Guidance: Clarifies that electronic informed consent (eIC) must comply with 21 CFR Part 11, and reaffirms the importance of real-time communication with participants.
Draft Version: Touched on eIC but did not provide robust regulatory linkage.
- Decentralized Drug Administration
Final Guidance: Offers greater clarity on shipping investigational products directly to participants, outlining conditions under which this is acceptable, including temperature control and accountability protocols.
Draft Version: Addressed this only briefly and lacked operational details.
- Early FDA Engagement
Final Guidance: Strongly encourages early interaction with the relevant FDA review division when planning to include decentralized elements, particularly for complex or first-in-human trials.
Draft Version: Recommended FDA communication but without stressing early engagement or outlining its benefits clearly.
These changes emphasize the FDA's increased focus on implementation clarity and regulatory flexibility.
The Cost Advantage: How DCTs Are Redefining Trial Economics
DCTs offer significant cost-saving potential across various trial stages:
- Slash Recruitment Expenses: Traditional trials incur high recruitment costs (travel, accommodation, advertising). DCTs lower these costs by allowing remote participation or local healthcare engagement, expanding access to underrepresented groups and improving efficiency.1,2
- Long-Term Financial Benefits: Beyond recruitment, DCTs reduce operational costs, including travel, site overhead, and patient compensation, by enabling real-time data collection and minimizing in-person visits. This results in quicker trial completion, faster market access, and reduced financial expenditure.3
- Efficiency in Data Capture: DCTs leverage remote technologies to capture data continuously, reducing the need for costly in-person visits. Automation further lowers manual data entry costs and increases trial efficiency.2
- Smart Cost-Saving Strategies: Using telemedicine, mobile units for remote visits, and cloud-based platforms for data analysis helps reduce expenses. Hybrid models, combining in-person and remote visits, allow for better resource allocation and reduced site-based costs.1
- Impact on Site Monitoring Costs: Remote monitoring through telemedicine platforms reduces the need for frequent on-site visits, cutting site monitoring costs. Digital tools to ensure data accuracy provide cost-effective oversight while maintaining data integrity.3
Real-World DCT Success Stories: Proof Of Concept
Several pharmaceutical companies have successfully implemented DCTs, demonstrating the model’s effectiveness:
- Example 1: In a fully decentralized Phase 4 breast cancer trial conducted in collaboration between Science 37 and a top 10 global pharmaceutical sponsor in 2020, the study leveraged telehealth, mobile nursing, and ePRO tools to conduct trial visits and data collection entirely outside traditional sites. The decentralized approach led to a 96% patient retention rate, representing an estimated 30% improvement over traditional site-based oncology trials, where retention rates typically hover around 70%. The trial's design not only improved retention but also accelerated study startup timelines and reduced operational costs.5
- Example 2: Medable Inc. reported in 2022 that the use of decentralized clinical trial elements, including wearable devices for real-time patient monitoring, contributed to a 30–40% improvement in retention rates across multiple studies, including those involving rare disease populations. These findings suggest broader patient engagement and faster responsiveness to adverse events.6
Tackling Key Questions: What’s Next For DCTs?
- Impact on Patient Recruitment and Retention: DCTs offer flexibility and accessibility, reducing dropout rates and enhancing participant engagement by eliminating travel and allowing participation from home or local clinics.1
- Overcoming Implementation Challenges: Integrating DHTs, maintaining data integrity, and training staff to manage decentralized elements are key challenges, but these can be addressed through collaboration and innovation.3
- Enhancing Data Accuracy: DHTs ensure real-time data capture, minimizing errors and improving data accuracy, leading to more reliable trial results.1
- Role of KOLs and HCPs: KOLs and HCPs play a pivotal role in educating patients, promoting DCTs, ensuring adherence, and supporting the integration of these models in clinical practice. Their advocacy and expertise are key to DCT adoption.1
- Ensuring Safety and Integrity in Decentralized Trials: The FDA ensures safety by requiring robust data monitoring systems that track adverse events and maintain accuracy across decentralized sites. This ensures that decentralized trials uphold the same scientific rigor as traditional ones.1,3
A Call To Action: Be At The Forefront Of DCT Innovation
To stay at the forefront of clinical innovation, industry, KOLs, and HCPs should:
- Stay Informed: Stay updated on FDA guidance for DCTs and explore remote monitoring technologies.
- Collaborate with Industry Partners: Collaborate with your external partners on DCT implementation, or find new external partners who can, for smoother regulatory processes.
- Train and Educate Teams: Ensure your clinical staff is well versed in DCT technologies and methodologies. Specialized training in medical and regulatory writing can streamline documentation and reporting.
- Adopt DCTs in Research: Integrate decentralized elements into future clinical trials, considering both fully decentralized and hybrid models based on trial needs.
While DCTs offer numerous advantages, managing them across multiple locations and integrating digital tools present challenges. However, the benefits, such as faster data collection and more diverse patient engagement, present significant opportunities for innovation. By embracing DCTs, industry can generate robust clinical evidence more efficiently, accelerating the development of treatments.3
Conclusion: A Transformative Future For Clinical Trials
The FDA’s 2024 guidance on decentralized clinical trials is not just a regulatory update but a comprehensive road map for the future of clinical research. By emphasizing patient centricity, accessibility, and advanced digital tools, DCTs promise to revolutionize trial design and execution for more personalized care and faster drug development. Industry, KOLs, and HCPs have the opportunity to lead this transformation, shaping a future where clinical trials are more accessible, efficient, and reflective of diverse patient populations.
As DCTs become the new norm, collaboration, compliance, and safety will be paramount in ensuring their success and expanding their impact on the global healthcare landscape.
References
- U.S. Department of Health and Human Services, Food and Drug Administration. (2024). Conducting clinical trials with decentralized elements: Guidance for industry, investigators, and other interested parties. U.S. Food and Drug Administration. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/conducting-clinical trials-decentralized-elements
- Nebel R. Decentralized clinical trials and the future of drug development. Available at: https://phrma.org/Blog/Decentralized-clinical trials-and-the-future-of-drug-development. Published Aug. 9, 2023. Accessed April 2, 2025.
- Joseph M. Unger. Decentralized clinical trials: FDA guidance and the ‘new modus operandi’. Available at: Decentralized clinical trials: FDA guidance and the ‘new modus operandi’. Published Jan. 30, 2025. Accessed April 2, 2025.
- Understanding the New FDA Guidance on Decentralized Clinical Trials. Decentralized Clinical Trials: The New FDA Guidance Analyzed. Published Jan. 13, 2025. Accessed April 3, 2025.
- Science 37. (2022). Agile in Action: Science 37 succeeds delivering a hybrid oncology trial. Science 37. https://www.science37.com/sites/default/files/2022-07/S37_WhitePaper_AnExecClinicalTrialPlaybook_July2022.pdf
- Medable Inc. (2022). Decentralized trials: The future of clinical research (White paper). Medable. https://www.medable.com/resources/whitepapers
About The Author
 Balaji Maddiboyina, Ph.D., is a senior MLR specialist — medical reviewer at Freyr Solutions with 13+ years of experience in scientific writing. He has a background in developing complex manuscripts in biomedical engineering, drug delivery, biomaterials, and nanotechnology with novel therapeutics to bring to market advanced medication systems for unmet medical needs.
Balaji Maddiboyina, Ph.D., is a senior MLR specialist — medical reviewer at Freyr Solutions with 13+ years of experience in scientific writing. He has a background in developing complex manuscripts in biomedical engineering, drug delivery, biomaterials, and nanotechnology with novel therapeutics to bring to market advanced medication systems for unmet medical needs.
