Designing Patient-Centric Recruitment Pathways With Real-Time Adherence Insights
By Monica Nandagopal, senior analyst – Pharma R&D, Beroe Inc., and Anthony Londino, CEO and founder, Med-Con Technologies

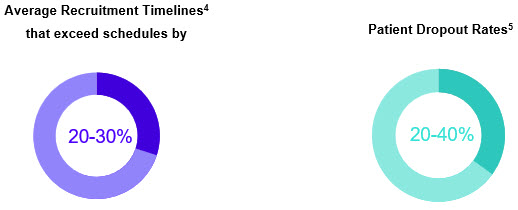
Patient recruitment and adherence represent critical bottlenecks in global clinical trials, accounting for more than 30% of study delays. Approximately 30% of participants drop out during studies, affecting timelines and data quality.1
The integration of digital adherence solutions capable of real-time monitoring and site-level oversight has shown measurable improvements in patient engagement and protocol compliance. These capabilities provide procurement teams with the evidence required to align partner selection with patient-centric goals.2
The Procurement Imperative In Clinical Trials
Effective management of pharma stakeholders is crucial to overcoming recruitment challenges and accelerating trial timelines.2,3 The expanding supplier ecosystem spans recruitment vendors, digital health platforms, CROs, and logistics partners.
Recent findings emphasize the significance of adherence tools:
- Digital adherence platforms consistently achieve >90% compliance compared to 64%–78% in manual paper-based systems.
- Real-time insights help prevent deviations by alerting teams before missed doses or delayed reporting escalate into compliance issues.
Procurement’s critical impact is as follows:
Designing Patient-Centric Recruitment Pathways
Patient centricity in clinical recruitment refers to designing trial pathways that prioritize accessibility, trust, and convenience for participants.6
Key components of an effective patient-centric recruitment include:
- Patient Understanding
- Behavioral analytics and social listening tools improve understanding of patient motivations for and barriers to enrollment.
- Adherence analytics add a complementary layer by identifying where patients struggle with daily study requirements, enabling tailored interventions early in the recruitment journey.7
- For example: An NIH-funded study by the University of South Carolina uses OpenClinica Recruit’s patient engagement platform and provides targeted digital ads focused on ethnicity, geography, and demographics via multiple channels. This approach increased recruitment speed by 10 to 15 times.8
- Technology Enablement
- Digital tools enable remote enrolment, data capture, and monitoring. Platforms that capture daily dosing behavior help recruitment teams identify which patient segments may require additional support informing more personalized messaging, follow-up strategies, and resource allocation.9,10
- For example, Lifebit’s digital patient recruitment case study demonstrates how integrating AI-driven outreach and real-time analytics streamlined enrollment. Their system facilitated faster identification of eligible patients and customization of outreach messaging.11
- Supplier Collaboration
- Recruitment vendors and engagement partners play a vital role in reaching and supporting diverse populations.
- When vendors have integrated adherence dashboards, sponsors gain greater visibility into site-level performance, enabling rapid adjustments to recruitment tactics and engagement touchpoints.12, 13, 14
- Procurement’s Role:
- Procurement ensures supplier strategies align with diversity, compliance, and timeline objectives.
- Outcome-based contracts can incorporate adherence thresholds (e.g., >90% compliance, timely data submission), ensuring suppliers remain accountable for the patient experience throughout the recruitment and retention life cycle.
By integrating these components, patient recruitment models promote equal access to all patients, driving higher recruitment efficiency and retention.15, 16 The flowchart below depicts the components of a patient-centric recruitment framework:
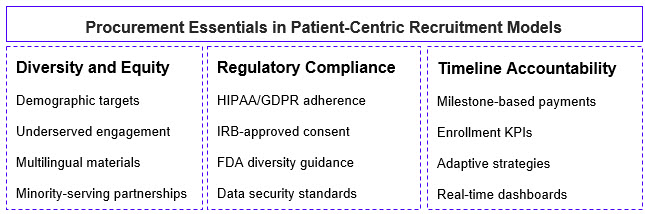
Sources: Applied Clinical Trials, Frontiers, Science Direct
Embedding Real-Time Adherence Insights
Real-time adherence refers to the continuous capture of patient dosing, visit compliance, and symptom reporting via digital tools.
The advantages are significant:
- Early detection of at-risk patients enables timely interventions.
- Higher data quality due to fewer missing or delayed entries.
- Improved site oversight, especially in multi-country studies.
- Operational efficiency, with reduced reconciliation workload.
These outcomes highlight that adherence insights are not operational aids; they shape recruitment strategy, site selection, and resource prioritization.17
Procurement teams can leverage adherence insights by integrating adherence-related key performance indicators into vendor contracts.
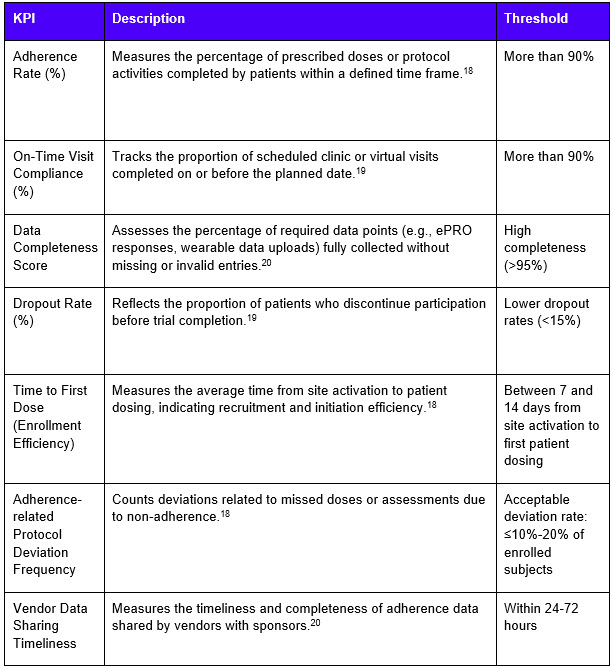
Overall, combining patient recruitment with adherence monitoring creates recruitment efforts that are more targeted and patient-centric.18
Integrating Supplier Insights From Selection To Partnership
Sponsors should leverage supplier insights such as benchmark data, technology capabilities, and past vendor performance to make informed decisions.
Key dimensions for supplier evaluation include:
- Patient Recruitment Conversion Rates: Metrics indicating how efficiently a supplier converts candidate patients into enrolled participants, reflecting recruitment success.21
- Use of Digital Engagement Tools: Evaluation of suppliers’ adoption of digital platforms like AI-driven outreach, mobile apps, or telehealth solutions.22
- Diversity Outreach Capabilities: Assessing vendors' ability to reach diverse patient populations through culturally sensitive programs and partnerships.23
- Adherence Monitoring Infrastructure: Adherence monitoring maturity, including real-time dashboards, dose-tracking systems, and data-sharing reliability.17 Real-time patient adherence monitoring data is typically built into an adherence monitoring solution at no extra cost.
Studies comparing digital vs. manual adherence management consistently demonstrate strong correlations between real-time visibility and reduced dropout rates, lower deviation frequency, and faster titration cycles. Procurement teams can leverage these benchmarks to prioritize suppliers with digital readiness.2
As per a recent study conducted by Med-Con Technologies, real-time adherence monitoring has demonstrated the ability to elevate compliance rates above 90%, reduce administrative burden, and enable faster, safer dose-related decisions compared to traditional paper-based approaches
Building A Procurement Framework For Patient-Centric Trials
By embedding real-time adherence data into sourcing models, sponsors can align supplier incentives with trial success metrics such as recruitment speed, patient diversity, and long-term compliance.
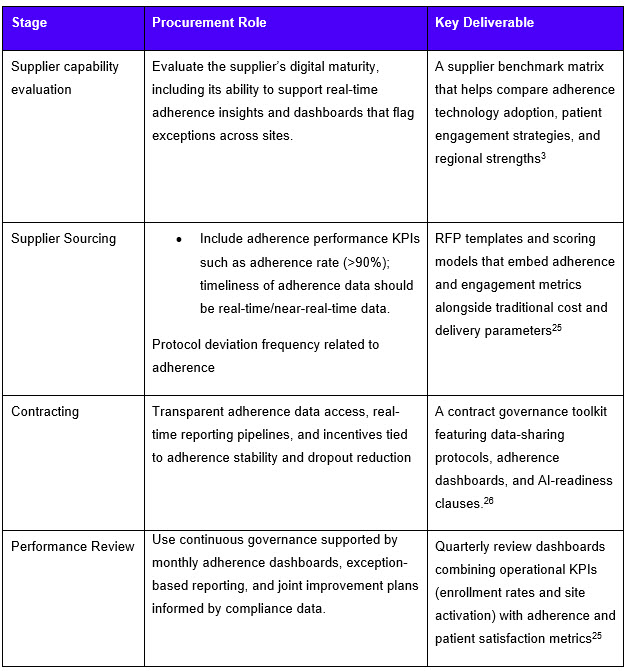
To enable effective patient-centric recruitment with patient engagement and adherence data as KPIs, sponsors should:
- Enable Cross-Functional Governance: Integrate adherence data into joint decision-making across procurement, clinical operations, and suppliers.
- Expand the Definition of Supplier Performance: Include adherence, retention, and data-timeliness as KPIs to evaluate suppliers on their ability to maintain patient participation and minimize dropouts across trial phases.
- Leverage Data for Predictive Procurement: Integrate predictive analytics and adherence trend analysis to forecast dropout risk and inform sourcing decisions.
- Drive Continuous Improvement Through Data Transparency: Mandate real-time adherence dashboards and automated reporting pipelines.
- Foster Outcome-Based Partnerships: Link incentives to adherence stability, deviation prevention, and patient retention.3, 25, 26
As adherence analytics mature, procurement is uniquely positioned to orchestrate an ecosystem of technology and service partners capable of delivering patient-centric value. Real-time adherence systems have demonstrated tangible improvements in compliance, dosing accuracy, and monitoring efficiency. From the beginning and throughout the trial, key stakeholder and patient buy-in, as well as well-executed planning, is essential.
The next frontier in patient-centric trial management lies in the fusion of AI-driven risk modelling, real-time adherence systems, and predictive supplier performance scoring. By leveraging these capabilities, sponsors can identify adherence risks at the study or site level.10 Procurement’s influence will extend beyond managing supplier costs to shaping pathways through which patients engage with clinical research, contributing to trial success and long-term healthcare impact.3
References:
- P. Subramani, A. Ranjit Mohan, A. Ramasamy, U. Ranjit, K. M. Venkat Narayan, K. A. Mohammed, K. Kulasegaran and V. Mohan, “Strategies for participant retention in long term clinical trials: A participant –centric approaches,” National Center for Biotechnology Information, 2022.
- “The Evolution of Procurement in Business Strategy,” 20 January 2025. [Online]. Available: https://procurementandsupplychainmanagement.co.uk/procurement/f/the-evolution-of-procurement-in-business-strategy.
- “Credevo,” 15 12 2023. [Online]. Available: https://credevo.com/articles/2023/12/15/the-complete-guide-to-clinical-trial-sourcing-key-strategies-best-practices/.
- A. Laurent, “Intuition Labs,” 12 11 2025. [Online]. Available: https://intuitionlabs.ai/articles/clinical-trial-delays-phase-i-iii.
- B. Debdipta, S. Shruti, S. Unnati, K. Harshad, M. T. Urmila and N. J. Gogtay, “Factors influencing recruitment and retention of participants in clinical studies conducted at a tertiary referral center: A five-year audit,” National Center for Biotechnology Medicine, 2020.
- K. J, I. T van, V. D de, D. Y and K. I, “Healthy participant engagement in early clinical trials: results from the European EUFEMED survey,” National Center for Biotechnology Information, 2025.
- A. Annick, B. Jasmine and G. Ken, “Using Patient Advisory Boards to Solicit Input Into Clinical Trial Design and Execution,” National Center for Biotechnology Information.
- “Open Clinica,” 15 January 2025. [Online]. Available: https://www.openclinica.com/blog/clinical-trial-patient-recruitment-two-case-studies/.
- K. Amy, M. Jennifer, Y. Joshua, C. Raphael E, M. Tiana J and M. Tim K, “Digital technologies used in clinical trial recruitment and enrollment including application to trial diversity and inclusion: A systematic review,” National Center for Biotechnology Information, 2024.
- “Antidote,” [Online]. Available: https://www.antidote.me/blog/5-clinical-trial-digital-recruitment-services-to-consider.
- “Trial and Digital Error? A Clinical Recruitment Case Study,” 30 July 2025. [Online]. Available: https://lifebit.ai/blog/clinical-trial-recruitment-digital-results-case-study/.
- Avacare, “Applied Clinical Trials Online - Clinical Trial Diversity in Action: A Site Roadmap for Sponsors and CROs,” 30 October 2024. [Online]. Available: https://www.appliedclinicaltrialsonline.com/view/clinical-trial-diversity-in-action-a-site-roadmap-for-sponsors-and-cros.
- P. Shilpa and D. Jeroze, “Revolutionizing patient recruitment experience in trials using the design-thinking framework,” National Center for Biotechnology Information, 2025.
- Y. Kay Por, G. Simon, S. Catherine, W. Jeremy, M. Shyam, H. Syed Sa, R. Farrukh, S. Natasha, T. Joyce, T. Julie, C. Brendan, F. Kay, B. Simon, P. Dhruv and D. Davinder P S, “Increase in recruitment upon integration of trial into a clinical care pathway: an observational study,” National Center for Biotechnology Information, 2021.
- S. Wolfgang, N. Carsten, O. Yulia and J. Chittaranjan, “BCG: Two Keys to Success in Supplier Risk Management,” 23 September 2023. [Online]. Available: https://www.bcg.com/publications/2023/how-to-mitigate-supply-chain-risk.
- “N-SIDE: The Secrets of Vendor Collaboration: Enhancing Your Clinical Supply Chain,” 23 October 2024. [Online]. Available: https://www.n-side.com/en/insights/the-secrets-of-vendor-collaboration-enhancing-your-clinical-supply-chain/.
- “Medable: Leveraging eCOA to improve patient adherence in clinical trials,” 3 Feb 2025. [Online]. Available: https://www.medable.com/knowledge-center/guide-leveraging-ecoa-to-improve-patient-adherence-in-clinical-trials.
- “Mahalo Health: Improving Adherence and Protocol Compliance in Clinical Trials,” 2 June 2025. [Online]. Available: https://www.mahalo.health/insights/how-to-increase-adherence-and-protocol-compliance-for-clinical-trials.
- S. Azfar-e-Alam, S. Alla, W. G. Charles and G. Barbara, “Early Participant Attrition from Clinical Trials: Role of Trial Design and Logistics,” National Center for Biotechnology Information.
- “Trial X: How eCOA Benefits Patients, Sites and Sponsors,” 30 June 2025. [Online]. Available: https://trialx.com/ecoa-clinical-trials-benefits/.
- “One Study: 6 Key Clinical Trial Metrics to Evaluate Your Patient Recruitment Campaigns,” 20 May 2024. [Online]. Available: https://blog.onestudyteam.com/clinical-trial-metrics-to-evaluate-patient-recruitment-campaigns.
- K. Amy, M. Jennifer, Y. Joshua, C. Raphael E, M. Tiana J and T. K. Mackey, “Digital technologies used in clinical trial recruitment and enrollment including application to trial diversity and inclusion: A systematic review,” National Center for Biotechnology Information, 2024.
- T. Tosin, W. Erin, M. Hailey N, O. Oluwabunmi, B. Samuel, P. Timothy and C. R. Himmelfarb, “Leveraging digital tools to enhance diversity and inclusion in clinical trial recruitment,” Frontiers, 2024.
- Expert Response: Real-Time Adherence Tracking in a Global Cardiology Trial: How MedCon Improved Compliance and Oversight in a Complex Dosing Study, 2025.
- “Endpoint Clinical: Clinical Trial Governance A Strategic Look at KPIs from an RTSM Perspective,” [Online]. Available: https://www.endpointclinical.com/insights/clinical-trial-governance-a-strategic-look-at-kpis-from-an-rtsm-perspective.
- “Procure Desk: Clinical Trial Procurement,” 15 October 2024. [Online]. Available: https://www.procuredesk.com/glossary/clinical-trial-procurement/.
About the Authors:
 Monica Nandagopal is a senior analyst – Pharma R&D – Clinical and Preclinical Research with Beroe, Inc. Monica is a category research analyst with over six years of experience in market research and consulting. Her insights have enabled top pharma companies in their strategic decisions on supplier outsourcing, category management, and planning.
Monica Nandagopal is a senior analyst – Pharma R&D – Clinical and Preclinical Research with Beroe, Inc. Monica is a category research analyst with over six years of experience in market research and consulting. Her insights have enabled top pharma companies in their strategic decisions on supplier outsourcing, category management, and planning.
 Anthony Londino is the CEO and founder of Med-Con Technologies. Anthony has more than 30 years of leadership experience working with Fortune 100 companies; the last 12 were directly in the pharmaceutical clinical trial space.
Anthony Londino is the CEO and founder of Med-Con Technologies. Anthony has more than 30 years of leadership experience working with Fortune 100 companies; the last 12 were directly in the pharmaceutical clinical trial space.
