Digitally Enabled Clinical Trials: A Practical Guide
By Jennifer Lee, Elevar Therapeutics

The pandemic has accelerated the adoption of digital clinical trials. According to Oracle's November 2020 report, 76% (191.5/252) of survey respondents1 reported that their trials are decentralized. In 2021, the State of the Industry Survey2 showed that approximately 83% of sites and sponsors invested in decentralized study capabilities like remote monitoring. Not surprisingly, 80% of research sites expect this trend to continue.
Undoubtedly, digital trials will stay as the world co-exists with the pandemic as it does with flu, measles, and HIV. It is essential for both sponsors and sites to be early adopters of digital trials. This article will examine the benefits and challenges of digital clinical trials and identify key considerations for sponsors and sites as the world continues to navigate the new normal.
Benefits Of Digital Clinical Trials
Increased Diversity Will Improve Applicability of Study Results
Participant homogeneity plagues both the applicability of study results and the equity of research overall.3 In a recent report from the FDA, minorities are significantly underrepresented in clinical trials4. While people of color make up about 39% of the U.S. population, women, the elderly, and people of color, especially Black and Hispanic patients, represent 1% to 5% of patients in trials.4 Digital clinical trials can reach broader patient geographies across the globe and help build sites where the patients are, not just where the hospitals are.
Positive Patient Experience Will Drive Quality, Retention, and Enrollment of Clinical Trials
For patients who cannot visit a study site regularly, televisits, remote patient monitoring, and fully digital or hybrid clinical trials can be a great option. Investigators can deliver care to patients with greater convenience, with continuous monitoring of health that can improve patient engagement. Another potential benefit includes a faster recruitment rate as investigators can reach much broader populations of patients geographically.
Digital Clinical Trials: Challenges And Considerations
One of the challenges of digital clinical trials is that it may not be for every clinical trial. Think of it as not a one-size-fits-all model. For a clinical trial that requires a complex treatment procedure (e.g., assessing lymph nodes or prostate nodule, with normal PSA5), an in-patient visit may be a better choice rather than a televisit. To ensure success, the design and implementation should be customized to accommodate each clinical trial's needs.
Furthermore, regulatory bodies in each country have their own rules and opinions, so it is essential to understand regulators' positions and recommendations before investing in a digital solution for your trial.
5 Digital Trends To Know About
1. Fully Decentralized/Virtual Clinical Trials: Fully decentralized/virtual clinical trials provide opportunities for investigators and patients to meet virtually face-to-face for the entire clinical trial duration.
2. Telemedicine: Telemedicine provides opportunities for investigators to communicate, diagnose, or treat patients without being in the same room with patients, via phone calls, video conferences, emails, or text messages. Patients' perceptions of telemedicine have been shifting in a positive direction since the pandemic.
3. Hybrid Clinical Trial: Hybrid trials use mobile technology, local health care providers (HCPs), telemedicine, or other measures in conjunction with traditional in-person aspects of clinical trials. 6, 7 Electronic clinical outcome assessment (eCOA) and remote eConsent are examples of digital solutions used in hybrid trials. eConsent enables patients to consent from home and avoid unnecessary site visits, providing flexibility and real-time consent tracking.
4. Remote Patient Monitoring: Remote patient monitoring includes wearables, digital therapeutics, and biosensors. Several studies8 have shown that remote patient monitoring reduced 9 the risk of hospital readmissions by 76%. For example, long stretches at home between cancer patients' treatments may cause issues that require them to be hospitalized, which could have been avoided through proactive monitoring and management.10
5. The Direct-to-Patient (DTP) Model: In DTP, the study drug is shipped directly to the patient's home from a study site or depot. This home delivery (FDA Guidance) 11 ensures continued access to study medicine. However, if the product requires direct oversight by a healthcare provider (e.g., IV injection), this model may not be feasible. The DTP model could increase protocol compliance and higher retention.
A Practical Guide For Sponsors
As with all trials, building strategies for identifying and mitigating risks is recommended before investing in digital solutions. As a start:
- Determine if digital tools should be developed internally or outsourced.
- Assess what hurdles would need to be overcome to implement digital solutions.
- Evaluate what kind of backup plans and mitigation strategies are needed to avoid surprises.
- Ask whether the patient population is comfortable with remote patient monitoring.
- Ask how data quality will be tracked (authentication and data privacy) and who will own data.
- Consider that deploying many digital options may be counterproductive, as this would increase the patients' responsibility and inconvenience, impacting protocol compliance and data quality.
- Assess the regulatory acceptability of data/study results.
- Determine whether you have the right technology vendor now.
Considerations For Sites
It is essential for sites to evaluate their capability to conduct in-home visits or accommodate remote monitoring visits, as you might need to update the budget to implement these steps.
The following is a practical guide for implementing digitally enabled clinical trials:
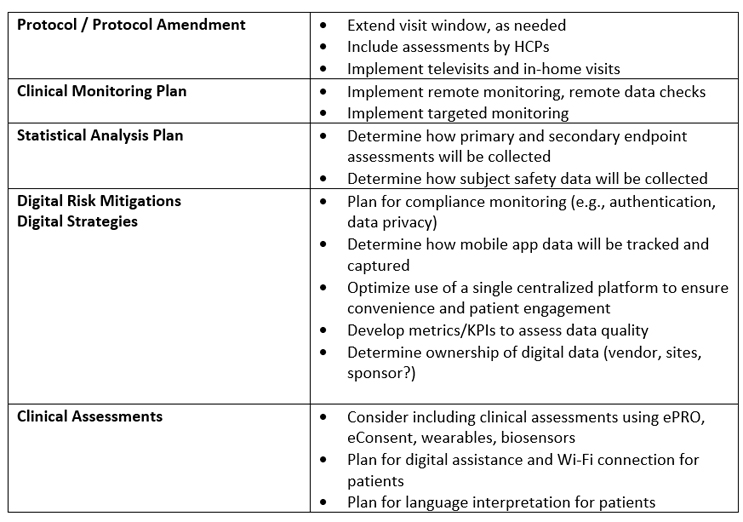
Conclusion
Adopting digital clinical trials can be daunting, but you can make this easy by taking a small step at a time. Using this practical guide, you can assess the benefits and obstacles of digital options and implement the one that is the best fit for your clinical trial.
References:
- The Accelerated Evolution of Clinical Trials in a Pandemic Environment, Oracle Research Report, November 2020 https://www.oracle.com/oce/dc/assets/CONT2CC43C146CD14B52A0A103ABD34D70BB/native/oracle-report-11-17-20.pdf?elqTrackId=9416c79188ad4c50a7bd8beb10c9837b&elqaid=103092&elqat=2
- www.florencehc.com/downloads/2021-state-of-clinical-trial-technology-industry-report/
- How Decentralized Clinical Trials Can Play A Role In Increasing Patient Recruitment And Diversity (forbes.com)
- As precision medicine grows, so does the importance of clinical trial diversity - MedCity News
- Where Telemedicine Falls Short (hbr.org)
- Food and Drug Administration, Center for Drug Evaluation and Research. CDER 2021 Guidance Agenda: New & Revised Draft Guidance Documents Planned for Publication in Calendar Year 2021. https://www.fda.gov/media/134778/download
- European Medicines Agency. EMA Regulatory Science to 2025: Strategic reflection. Published 2020. https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/ema-regulatory-science-2025-strategic-reflection_en.pdf
- Using Remote Monitoring to Reduce Hospital Visits for Cancer Patients (hbr.org)
- Remote Patient Monitoring Trends & Health Devices in 2021 (businessinsider.com)
- Characteristics of telehealth users in NYC for COVID-related care during the coronavirus pandemic | Journal of the American Medical Informatics Association | Oxford Academic (oup.com)
- download (fda.gov)
About The Author:
 Jennifer Lee is vice president of drug development at Elevar Therapeutics. An expert in advancing drug development pipelines from preclinical to commercialization, she has held positions in small, mid-, and large pharma/biotech companies and has managed product candidates for oncology, CNS, infectious/rare disease, and immuno-gene/cell therapies. She has successfully launched numerous products and turned around programs that resulted in licensing agreements and market registrations. Lee received a bachelor’s degree in biochemistry at the University of Illinois at Chicago and a master’s degree in clinical research and regulatory administration at Northwestern University.
Jennifer Lee is vice president of drug development at Elevar Therapeutics. An expert in advancing drug development pipelines from preclinical to commercialization, she has held positions in small, mid-, and large pharma/biotech companies and has managed product candidates for oncology, CNS, infectious/rare disease, and immuno-gene/cell therapies. She has successfully launched numerous products and turned around programs that resulted in licensing agreements and market registrations. Lee received a bachelor’s degree in biochemistry at the University of Illinois at Chicago and a master’s degree in clinical research and regulatory administration at Northwestern University.
