Digitizing The Clinical Protocol: Small Steps For Seismic Change
By Kelsey Jakee, PA Consulting, and Rob DiCicco, TransCelerate BioPharma, Inc.

We are in an era of clinical trial modernization. Decentralization and direct data capture contribute to the proliferation of platform systems, software, devices, and data sources used in a clinical trial. A “city of systems” usually requires bespoke integrations to exchange data which drives up cost, complexity, and cycle time. At the same time, efforts to further streamline development through platform trial designs, pragmatic or point-of-care trials, and data sharing or reuse are gaining traction to accelerate trials and harness the power of data. This carries some risk of introducing or worsening a so-called “digital divide” and places an increased technology burden on all stakeholders in the clinical research ecosystem.
These changes intersect with a unique challenge: reducing the friction for data exchange. This data exchange problem is notably present with information in the protocol, which forms the backbone of any clinical trial. Protocol information is needed by virtually every system used to plan, run, analyze, and report a clinical trial. Still, it is often trapped in a document that is not machine-readable (essentially, electronic forms of paper), requiring repeated human interpretation and data entry. Manual approaches like these stifle speed and limit innovation, making realizing the industry’s ambitions to modernize clinical trials more difficult.
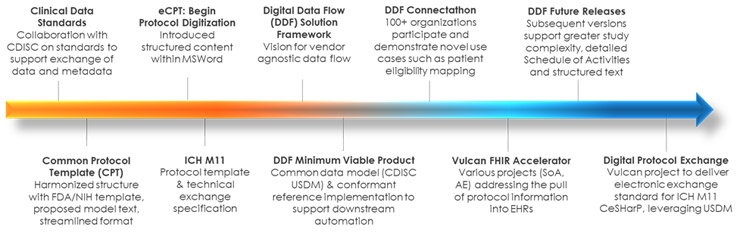
In recent years, TransCelerate BioPharma, in collaboration with CDISC, has made progress toward tackling this problem with the Digital Data Flow (DDF) Initiative, a program of work aimed at automating the exchange of protocol information across the study life cycle. The initiative has delivered foundational standards that create a common digital language for protocols that may be applied consistently across organizations. CDISC’s Unified Study Definitions Model (USDM) offers far greater machine-readable structure for protocol information than has ever existed in an industry-standard format. Alongside study and study design concepts, the model captures what patient data is to be collected, how, and on what schedule, and can accommodate a range of complex designs and study types.
Systems used to design studies and protocols can specify structured content using the USDM and exchange it more seamlessly with downstream systems requiring this information for study start-up and execution (e.g., EDC, eCOA) using standard application programming interfaces (APIs). Downstream system set-up thus can become more automated, with minimal manual intervention or human interpretation of information. Automated analytics are also made possible with this common structure for protocol information, with the potential to support capabilities such as AI/ML-assisted smart study design, budgeting, feasibility, patient eligibility matching, and patient burden scoring.
Taking a Pragmatic Approach
TransCelerate’s approach to Digital Data Flow is centered on three key philosophies:
- Show vs. Tell: Beyond supporting CDISC’s development of a new data model for protocol information, we have taken it further by offering a demonstrated mechanism to connect systems that produce protocol information and those that consume it. The Study Definitions Repository (SDR) Reference Implementation is a model approach to input, export, and store study designs in a USDM-compliant format, using CDISC-standard APIs for exchange with systems. The source code for the Reference Implementation is open-source for anyone to use freely, providing a vendor-agnostic, platform-independent tool for end-to-end digitalization. While using a stand-alone SDR within sponsor organizations is feasible and, in some cases, may be preferred, there are a variety of adoption patterns made possible with this model approach — including leveraging an existing metadata repository or other clinical software to perform the same functions of the SDR.
- Engage with Tech: Throughout the process, we are engaging with implementers in the technology community who play a critical role in applying standards in their software and digitalization roadmap. During the early stages, we engaged with eight technology providers offering study builder/protocol authoring tools or EDC software to help guide the development of data standards and SDR design. The ability to use a digital schedule of activities to automate EDC setup and reduce start-up time is a high-value early use case. As development progressed, we engaged over 100 organizations in a public Connectathon event to test or demonstrate possible applications of our solutions. Since the release of the USDM and SDR Reference Implementation, over 25 clinical technology organizations have used these solutions to publicly demonstrate a variety of use cases for study design, downstream automation, and analytics. Going forward, we are expanding our engagement in the clinical technology community ranging from operational systems to decentralized trial platforms to analytics suites. We encourage the continued development of new use cases, such as the ability to exchange inclusion and exclusion criteria with electronic health record systems to support recruitment.
- Cultivate Value Sustainably: The development and application of a standards-based approach to protocol digitization is an iterative process. With each successive release, we continue to pursue opportunities to align with related initiatives to unify, rather than proliferate, standards. Alignment with the ICH M11 Clinical electronic Structured Harmonised Protocol (CeSHarP) offers a future pathway to harmonized protocol structure and exchange with regulatory authorities. Compatibility with resources available through the Vulcan FHIR Accelerator will bridge the gap between traditional research systems and point-of-care systems. Furthermore, as the ecosystem of willing adopters grows around Digital Data Flow, we are preparing a roadmap to long-term, multi-stakeholder governance of solutions.
The foundational work for protocol digitization is rooted in TransCelerate’s Common Protocol Template Initiative beginning in 2014, which helped R&D organizations move toward greater harmonization in structure and data reuse customized protocols toward harmonization and reuse. It was informed through collaborative relationships with the FDA, NIH, CDISC, site-based organizations and PhUSE. Leveraging varied experiences and skillsets across these entities and looking through the lens of affected stakeholders were keys to success.
From Common Protocol Template to Digital Protocol Exchange
Considerations for Leaders
Clinical and technology leaders across both sponsors and sites will measure return on investment from protocol digitization in reduced cycle times, reduced cost, and net-new value creation for sponsors, sites, and patients from novel use cases (e.g., patient eligibility matching). Automation of data exchange will minimize time associated with manual curation, data entry, and system set-up during study start-up. Standards-based interoperability will provide greater flexibility to plug and play across various clinical systems in your IT landscape and beyond to point-of-care systems leveraged for research. This empowers companies to adapt quickly to change and collaborate more easily with research partners. Furthermore, the availability of protocol information in an industry-standard, digitized format will enable new innovations within the technology marketplace that can more easily cross organizational boundaries.
While big-picture value is evident, digital transformation is difficult for any company alone to embrace — particularly in healthcare and biopharma R&D, which has lagged behind other sectors. Decades of customization and bespoke implementations have led to a complicated network of incompatible systems that will continue to make data exchange difficult without a standards-focused approach. Leaders will be pressed to get more value out of the data and platforms they have already invested in so as not to limit or slow down programs already in flight. Given this, what can leaders do to prepare and make incremental steps toward transformation now?
First, it is important to clearly demonstrate the value of embracing protocol digitization in your business. Downstream automation of EDC configuration from a digital protocol specification is a ready-now use case, as evidenced by several EDC vendors who have already used the DDF standards and reference implementation to showcase this capability. Companies can take the opportunity to run a small-scale pilot for a selected study — perhaps even running a traditional EDC set-up in parallel with the more digital, automated approach — to demonstrate cycle time reduction and efficiencies gained.
Second, leaders will need a view of their current and desired future IT landscape to develop a roadmap for bringing various systems into conformance with industry standards. Transition to a standards-focused approach will likely be gradual, with value compounding over time as more systems conforming to the common standard come online. Systems already planned for replacement or re-evaluation in the near term should be considered first to capitalize on existing efforts to change.
Finally, the people and process change associated with protocol digitization cannot be ignored. Companies need a method of specifying protocols in a digital format — some will leverage study design and protocol authoring software (“digital-first approach”). In contrast, others may be set on applying AI and Large Language Model techniques to extract a digital specification from a document-based protocol. With either approach, new skillsets will be needed, and new workflows may emerge that disrupt the current approach to study design, protocol authoring, content management, and data management. Early awareness and education on available standards will help prepare organizations to operate using digital protocol specifications before any future regulatory requirements that may mandate using digital protocols and/or structured metadata in submissions.
About The Authors:

Kelsey Jakee, managing consultant with PA Consulting, has an established track record of leading and managing transformation in pharmaceutical R&D. Over the past 14 years, she has advised Top 20 BioPharma and industry consortia on clinical innovation, new service and business model development, R&D technology strategy, digital transformation, improving the patient experience, and leveraging novel data sources across preclinical, clinical, and drug safety. Her mission is to drive high-impact and lasting change in clinical research to help us all live our best lives.

Rob DiCicco, vice president, portfolio management at TransCelerate BioPharma, is responsible for TransCelerate’s initiatives focused on process harmonization. These initiatives include Digital Data Flow, Clinical Content & Reuse, the Real World Data Program, and TransCelerate’s collaboration with the HL7 Vulcan Accelerator. Rob joined TransCelerate from IBM Watson Health, where he was the Deputy Chief Health Officer. At IBM, he worked closely with software designers and developers to inform product roadmaps and conducted research to assess the performance of solutions applied in the e-clinical environment. Prior to joining IBM, Rob had a long career with GlaxoSmithKline, where he served in a variety of leadership positions. While at GSK, Rob was the Vice President of Clinical Pharmacology Sciences and Study Operations with a global footprint that included the US, UK, Australia, and China.
