Diversity In Clinical Research Execution And Participation
By Denise Calaprice, Ph.D., senior consultant, Avoca Group

The events of the past two years created an urgent and nonnegotiable imperative to increase diversity in clinical research: diversity both in how such studies are executed, and in the types of patients recruited for participation.
As we move forward, industry leaders must synthesize this experience and incorporate its lessons into the clinical operations culture — deciding how much executional diversity to retain, determining how we can maintain diversity in trial participants, and marshalling the motivation of clinical researchers to sustain the effort required for both. In the 2020 WCG Avoca Industry Survey, we began to explore industry respondents’ views on patient diversity in clinical research participation. In 2021, we furthered our research by gathering experiences and views from respondents in a wide variety of industry positions regarding the relative priority and drivers of patient diversity in clinical research participation, as well as their experiences with diversity in clinical research execution and their perceptions of how the two are related.
The Survey Respondents
In this article we want to compare the results from the 2021 survey to those from the 2020 survey. The 2020 survey was designed to study how the events of that year accelerated operational and study design innovations within clinical development, as well as how respondents perceived clinical trial quality and efficiency to have been impacted, how management practices were adapted, and whether respondents anticipated a return to pre-innovation approaches, or an acceleration of innovation, post COVID-19.
For the 2021 survey, we sent invitations to companies in our database, and we posted an open invitation on our website and via LinkedIn.
The 2021 respondents included 64 sponsor companies, 35 providers, and two academic research organizations. Approximately 38% held positions within clinical development/operations, 34% within quality assurance, and the remainder within a wide spectrum of management and functional roles. Thirty-seven percent of respondents represented companies that sponsored or conducted more than 50 clinical trials during 2021, and 20% represented companies that sponsored/ conducted fi ve or fewer. Most respondents worked for companies headquartered in the U.S., but approximately one-third represented companies headquartered in Northern/Western Europe (19%), Japan (5%), or other parts of the world (9%). Seventy-eight percent of the respondents resided in the U.S.
How Important Is Clinical Trial Diversity?
In both 2020 and 2021, respondents were asked how critical to the quality of clinical research they considered each of several different types of diversity among trial participants (Figure 1). On average, respondents in 2021 felt equally strongly about diversity with respect to race/ethnicity, gender, global/regional standards of care, and disease stage. All of these were thought by most to be at least somewhat important and by approximately one-third to be critically important. Respondents were more divided when it came to economic diversity and population type (rural, urban, suburban). Except in the cases of racial diversity and diversity in disease stage, respondents on average felt more strongly about diversity in 2020 than in 2021.
Perceptions Of Performance
On average, respondents felt their companies had a middling level of performance in the area of clinical trial participant diversity and that the rest of the industry was performing even more poorly. Performance, with respect to gender diversity and global/regional standards of care, was felt in 2021 to have been about the same as in 2020, and performance, when it came to diversity in disease stage, was felt to have improved. However, performance regarding racial diversity, economic diversity, and diversity in population type (rural/suburban/ urban) was, on average, rated more poorly in 2021 than it had been in 2020.
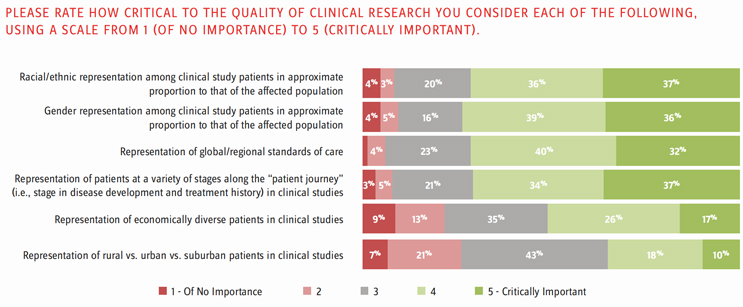
Figure 1. Importance of Diversity Among Clinical Trial Participants, Averages for 2020 -2021
Decentralized Clinical Trial Activities
On average, every aspect of decentralized clinical trials investigated in this survey was felt by respondents to have had a positive impact on clinical trial participant diversity in every respect. The tactics felt to have the most positive impacts were those that brought study-related medical care to convenient locations for participants: home healthcare provider study visits, point-of-care integration of clinical research (i.e., performance of trial procedures at usual points of care), study visits by telemedicine, and ship-to-home of clinical supplies. These were felt to be particularly impactful when it came to racial, economic, and population type (urban vs. rural) diversity. Online or wearable trial features (e-consent; wearables; patient portals, communities, or diaries; and completely siteless trials) were, on average, thought to be slightly less, but still positively, impactful.
Optimal Vs. Actual Level Of Diversity In Clinical Trial Execution
It was widely believed that diversity among clinical trial participants could be supported by the right level of flexibility in how clinical trials are designed and operationalized. With respect to specific operational approaches, respondents were most likely to report that their organizations were at the optimal levels of variability/ flexibility to support participant diversity when it came to studying staff-patient interactions, followed by overall operationalization tactics within an individual clinical trial (e.g., hybrid approaches to decentralization, patient engagement). Only about 1 in 6 respondents perceived their organization to have greater than optimal levels of variability/flexibility in any area. Variability and flexibility were most likely to be suboptimal in the areas of laboratory sample collection and overall operationalization of clinical trials across a development program (i.e., use of decentralized approaches for some trials). Among company types, provider organizations and top 20 pharma were generally most likely to be near optimal levels of variability and flexibility.
In conclusion, most respondents to the 2021 survey consider diversity among clinical trial participants to be important, and approximately 1 in 3 feel it to be critical to clinical trial quality. Despite this value, however, most respondents feel that their own companies’, and the industry’s, performance in this area could use improvement. Particularly, over the past two years, decentralized trial approaches have proven capable of ensuring and enhancing clinical trial performance in multiple ways. The promotion of diversity appears to be no exception, with every aspect of decentralized clinical trials investigated in this survey perceived by respondents to positively impact clinical trial participant diversity in every respect. Although some respondents felt that their companies had achieved the optimal levels of executional flexibility to promote participant diversity, at least in some operational areas, most respondents felt their companies had either too much or — more often — too little flexibility to support this goal.
