eConsent In Clinical Trials: Insights For Implementation During COVID-19 And Beyond
By Kelly Roland and Hannah Glenny, Otsuka Pharmaceutical

The world has changed in the wake of COVID-19. Clinical trial sites and sponsors are scrambling to adjust traditional clinical trial practices and processes to incorporate virtual interactions and data collection methods. A recent New York Times article titled “Telemedicine Arrives in the UK: 10 Years of Change in One Week” indicates a sharp, global change in acceptance of such methods. New mantras such as “flattening the curve” and “social distancing” require creative solutions and methods in relation to clinical trial models.
To adjust for these changes, clinical trial sponsors must adapt their protocols, and many are urgently doing so, to provide virtual tools for clinical trial use. One example of an important tool in this space is electronic consent, or eConsent.
Electronic consent enables sites to continue to consent and reconsent patients in clinical trials during this time of self-isolation. Not only does it allow patients to consent in the comfort of their home, but it also provides quantitative and qualitative data that isn’t available through traditional paper consent forms. Otsuka Pharmaceutical Development & Commercialization has been implementing eConsent over the past five years across multiple indications and phases of studies. The data collected from over 4,000 clinical trial participants globally permits knowledge sharing of best practices in this space. This article will share some insights on implementing eConsent in clinical trials.
With so many eConsent vendors out there, it is important to share a list of capabilities that are imperative to consider in your selection process. First, select a vendor that includes a video as part of the eConsenting process. The 5- to 7-minute videos typically outline key study procedures or expectations of the clinical trial participant in an engaging way that supplements the consent document. Otsuka’s internal data analysis indicates that 73 percent of participants watched at least 80 percent of the video, demonstrating its value in the process. Additionally, there was a correlation between those who spent more time on the video and those who completed the study, suggesting that watching the video may help improve retention rates. It is therefore recommended that you not allow a skipping function within these videos.
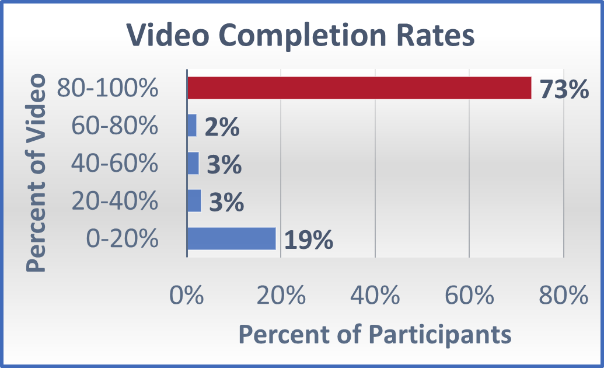
Using knowledge checks, or short quizzes, also helps ensure the potential subject has adequate information to make an informed decision about participating in a clinical trial. Collection of this data through eConsent provides sites and sponsors with evidence that participants are adequately informed when they consent to the clinical trial. Reviewing knowledge check questions in real time will identify questions frequently answered incorrectly and could indicate where further explanation is needed in the information being delivered.
We also recommend integrating eConsent with your electronic data capture (EDC) or eSource solution to alert sites when a participant is missing a countersignature or needs to be reconsented due to protocol changes. Additionally, it is preferable to receive near real time data from the eConsent vendor. From an operational perspective, it is imperative to have a plan in place to address any missing data, including checks or flags that will not allow a patient to move forward in the trial without the proper consent in place.
When implementing an eConsent in your trial, consider the differences among populations. Our data suggests that the amount of time a video is watched varies largely by age and phase, with an overall decrease in watch time as age increases and a higher watch time in Phase 2 trials.
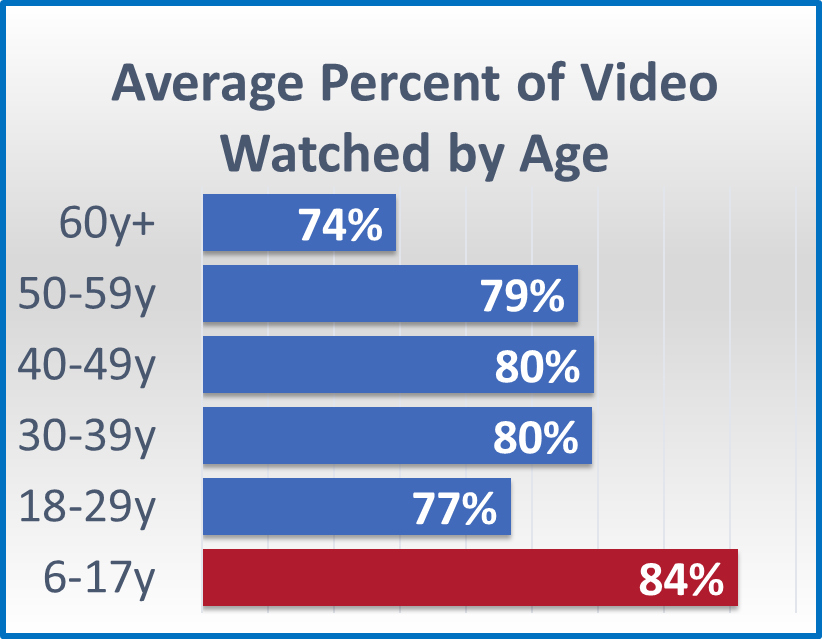
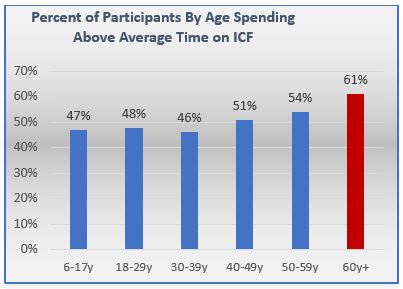
We also looked at how long participants spent on informed consent forms, taking into consideration that each study informed consent form is different. The time spent on the form was normalized using the average read time per study, and participants were classified as above or below their study’s average. The percentage of participants classified as spending an above average amount of time on their study’s informed consent form increased with age. These results indicate younger participants and/or their guardians spend more time on the video, while older participants spend more time on the informed consent form.
Diagnosis group also influences video watch time. For example, participants with mental illness watched significantly more of the video than those without. This information can be used to indicate which populations may require additional support with eConsent.
Because eConsent is technology driven, it allows sites to obtain information that typically is not available through a paper process. Having access to detailed information about the patient experience is critical to improving and enhancing the trial process. While the use of eConsent is highly beneficial during this pandemic, its value should be recognized as clinical trials return to normal — whatever that may look like.
About The Authors:
 Kelly Roland, MS, is an associate director in the Applied Innovation team at Otsuka Pharmaceutical, driving both innovative ideas and process improvement activities across all levels of the Otsuka portfolio. With an undergraduate degree in psychology and a master’s degree in innovation, she has participated in and overseen a unique set projects over the past 18 years. Her areas of expertise include change management, innovation strategy, process improvement, and customer engagement.
Kelly Roland, MS, is an associate director in the Applied Innovation team at Otsuka Pharmaceutical, driving both innovative ideas and process improvement activities across all levels of the Otsuka portfolio. With an undergraduate degree in psychology and a master’s degree in innovation, she has participated in and overseen a unique set projects over the past 18 years. Her areas of expertise include change management, innovation strategy, process improvement, and customer engagement.
 Hannah Glenny is a research associate in the Clinical Management and Applied Innovation team at Otsuka Pharmaceutical. Her most recent work includes leading various transformation initiatives to deliver the next generation of clinical research, while driving improved patient outcomes and operational efficiencies in the clinical trial process. She has a bachelor of science degree in psychology with a concentration in neuroscience and is actively pursuing a master of public health degree.
Hannah Glenny is a research associate in the Clinical Management and Applied Innovation team at Otsuka Pharmaceutical. Her most recent work includes leading various transformation initiatives to deliver the next generation of clinical research, while driving improved patient outcomes and operational efficiencies in the clinical trial process. She has a bachelor of science degree in psychology with a concentration in neuroscience and is actively pursuing a master of public health degree.
