Enforcing A Data Strategy In Clinical Research
By Hugh Donovan, BSc, Managing Expert, Clinical Center of Excellence

The Call to Action
Data is the most important asset for a pharmaceutical or biotechnology company to produce during clinical development. It is the foundation upon which everything else is built — without it, decisions cannot be made, and new treatments cannot be approved.
Therefore, efficiently acquiring high-quality data in a clinical trial should be a very high priority for the industry. The need to focus on a data strategy is increasing due to changes in the external environment as technology is constantly evolving. Electronic data capture (EDC) is now the normal way to way to capture case report form (CRF) data, and electronic patient reported outcomes (ePRO) is established as the approach to capturing patient-reported data. Additionally, automatic data capture through devices is expanding, and direct data capture from electronic health records is emerging.
In addition to technology, processes are changing with centralized monitoring, risk-based quality management, and, most recently, decentralized trials, all requiring re-evaluation processes. Trial designs are also evolving. Adaptive trial designs are now commonplace, requiring access to high-quality data throughout the study in order to decide rapidly on trial modifications. It also requires a very agile approach to study execution in order to implement study design changes on the fly. Recently, platform, umbrella, and basket trials were implemented, further stressing the infrastructure. With multiple trials conducted within one protocol, novel process development to handle the complexities became necessary.
There are multiple opportunities to capture data quickly, therefore accelerating drug development, but there is little evidence of clinical trial execution improving while cycle times are decreasing. Most companies lack an effective data strategy, as they do not build a solid foundation. An example of this is adopting an electronic data capture (EDC) technology. There is no evidence of reduced database lock times due to EDC. Many companies implemented an EDC without a comprehensive strategy. Data entry backlogs at the site did not decrease – in fact, in some cases, they increased. Not all checks were carried out in real-time as promised. Investigator signatures on case report forms (CRFs) are now on the critical path to database lock. These challenges are manageable, but there needs to be a well-thought-out strategy to do so, not a reliance on technology and assumptions.
When taking advantage of the multiple opportunities to improve efficiency and productivity through optimal data management, it is imperative for companies to develop a data strategy with mechanisms in place to maximize effectiveness once implemented.
The Scope of a Data Strategy
A successful data strategy needs to be comprehensive and detailed, covering every aspect of data management, from Phase I through Phase IV. It is not just about technology – a case in point is the poor initial implementation of EDC described above, which was often just about the technology, not about the impact on people and processes, all the way from the investigator’s site to database lock.
Our approach to developing the data strategy and ensuring compliance is to break it down into five equally important components: processes, resources, performance monitoring, tools, and information architecture.
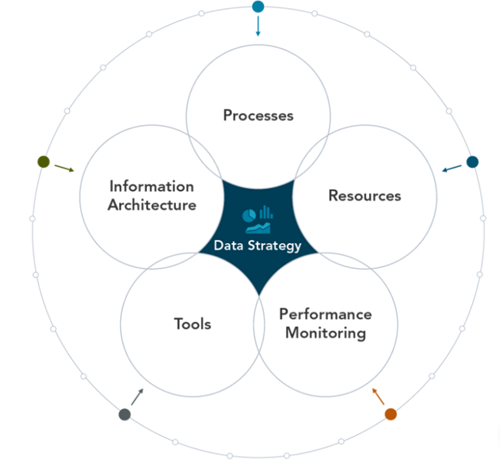
Development Methodology
Developing a comprehensive data strategy is a major undertaking, requiring dedicated resources from multiple functions. This is especially important if strategy development is used as an opportunity to enhance existing components – for example, developing new data standards or introducing a master data repository. Best practices recommend implementing a strategy based on current processes and systems, updating it as gaps are filled and new solutions are implemented. Delaying its implementation while waiting for major changes means both momentum and short-term opportunities for increased efficiencies are lost.
The development organization’s strategic objectives should include developing a data strategy. As such, senior executives should fund and oversee these objectives at a high level. Additionally, create a cross-functional task force with various stakeholder memberships.
To expedite the development of the strategy and to bring in external expertise, select an outside vendor to manage the process and to assist in the document writing to minimize project continuity impacts.
Governance
Once the task force completes its work and the data strategy is implemented, set a governance structure in place through the data management group reporting up to the data strategy owner. Various governance bodies should also receive authority to enforce the data strategy. For example, if data standards aren’t adhered to, there’s no value to having these standards. The data strategy should describe the governance structure’s organization in depth.
Risk Management
The external environment can change unexpectedly and rapidly – an example of this is when a currently approved vendor must be replaced due to a failed audit or a business failure. Without a clear, comprehensive, data strategy, which describes the process for handling these situations, there will be lengthy delays, while the response is discussed and a process is developed ad hoc.
Conclusion
Every pharmaceutical, biotechnology, and contract research organization (CRO) should implement a data strategy if there isn’t one already in place. Those with a data strategy in place should consider potential enhancements. While both pose a major undertaking for an organization, if executed successfully, it will:
- Increase efficiency through data standardization from set-up to reporting
- Accelerate decision making driven by centralized policy access
- Ensure a consistent approach to regulatory compliance
- Introduce a standard and cost-effective approach to evaluating and implementing new processes and technologies
- Measure data management process and system effectiveness through clear and relevant key performance indicators (KPIs)
- Improve organizational discipline, enabling growth
