FDA Issues Bioresearch Monitoring Technical Specifications Document To Help You Organize Your Submission
By Peter H. Calcott, Ph.D., FRSC, president and CEO, Calcott Consulting LLC

In September 2024, FDA issued the latest version of Bioresearch Monitoring Technical Conformance Guide: Technical Specifications Document: version 3.1.1 The original version was issued in late 2017, with substantial changes in 2020 and 2022. This version is probably going to be in place for a significant length of time. It describes in detail the structure of the Bioresearch Monitoring (BIMO) section of the submission. It is a road map for the construction of this part of the NDA or BLA that is submitted for approval and licensure of products submitted to CDER. While it does not mention CBER, I am sure the same principles apply for submission to that part of FDA. Why is this document important? In short, this part of the submission is a major communication tool within your submission to the FDA related to the mechanics and structure of the data related to your clinical trial section of the Common Technical Document (CTD)2 – Module 5.
NDAs and BLAs are very large submissions with much cross-referencing between sections and large volumes of raw data. The clinical module (5) is by far the largest part of a submission containing all things clinical: the studies and protocols and ancillary documentation, the data for efficacy, the safety data obtained from the trials, the analysis of the data with its statistical evaluation and the claims being made. All of this section must reference all data, making it transparent for review by FDA. With the advent of the CTD in 2000 (M4),2 the way we submit radically changed. The structure of the submission was standardized and would become universal over the next 20 years. With that standardization came the use of common electronic standards for file format and folder structure (M2).3 We are now into fully electronic submission in most markets (M8),4 including the U.S. This FDA document gives you the details you need to make sure the BIMO part of your submission is accurate. I have been involved in submissions for over 40 years, so I have seen the old and new ways of submitting and, in fact, the clinical part is the most complex and fraught with potential inaccuracies.
After submission of the NDA or BLA and before approval, there are BIMO inspections where the FDA will examine documentation of your studies, digging deep into specific studies. The purpose of this evaluation is to assure that the data submitted is supported by raw data at the source. You could consider it assuring data integrity. Accomplishing this inspection requires that the FDA investigators assigned to review data can find the raw data for each study they are evaluating. In contrast, in the case of a pre-approval or pre-licensing inspection (PAI or PLI) for the CMC part, raw data are found at the manufacturing site (CMO or company site) and corporate headquarters for certain elements, e.g., often pharmacovigilance. It is relatively straightforward. In the case of BIMO, data are not so conveniently located.
The numbers of clinical trial studies are many in a single submission. These include Phase 1 safety, Phase 2 safety and dosing studies, and Phase 3 efficacy pivotal studies. While a Phase 1 study might be performed at a single site, large Phase 3 trials involve many clinical sites (it can be the hundreds). Because of this, there are many principal investigators at these sites. In addition, the number of patients with their own personal data sets is often very large. In some vaccine trials there can be 30,000 patients. A large number of companies also outsource many of the activities associated with running a clinical trial to CROs. Raw data and documentation for specific activities are located at each of these individual sites (the many clinical sites, the CRO, and corporate). Thus, any single study might have raw data at any one of these three types of sites or even all of them. The purpose of this FDA document is to define and clarify how we as drug developers characterize where data is housed for each study, each patient, and each activity. If accurately categorized and documented, this helps FDA to determine where to send their investigators to evaluate any particular study to get the whole view of that study, either in person or electronically. They know what will be at that particular site and what will not be there.
This technical document provides the current FDA specifications, recommendations, and general considerations for preparing and submitting Clinical Study-Level Information, Subject-Level Data Line Listings by Clinical Site, and a Summary-Level Clinical Site Data Set that are used by CDER for planning of BIMO inspections in electronic format for NDAs, biologics license applications (BLAs), and NDA or BLA supplements. Readers are advised to check various FDA publications for the latest guidance.5
The technical document is broken down into four major sections, with four appendices linked to the appropriate sections.
The Clinical Study Level Information Section
This section outlines the comprehensive list of all clinical sites used in the submission. The primary ones will be for the pivotal studies, but others should be listed indicating what studies were conducted at which site. In Appendix 1, the agency illustrates one format for submission in the PDF format. It serves to describe the level of detail required. Alternative layouts are permissible. They also recommend that each study be included as a separate file.
Table 1 illustrates the details needed for the study file. Each study would have a discrete protocol that should be defined. There should be a clear indication whether a site was active throughout the study or whether it was terminated.
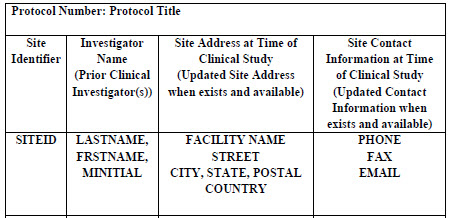
Table 1: Study Site Listing. Part of Table A of technical document.
Within another file the agency recommends that the applicant should indicate the location of each type of document together with a statement whether it was generated by the sponsor or the CRO. The documents include, for example, monitoring plans and reports, training records, and data analysis plans (e.g., items that some applicants organize in a Trial Master File). When locations are finalized, contact information must be provided. In some cases, the information may not be available at the time of writing, so it should be updated in a timely fashion.
The protocol and protocol amendments, with associated versions of the case report form, and the final version of the annotated case report form (case report form containing Clinical Data Interchange Standards Consortium and Study Data Tabulation Model (SDTM) annotations) are generally included in Appendix 16 of the Clinical Study Report or in the data sets folder for each study. It does not have to be repeated in both places but do note where it can be found. All versions of these documents should be provided, indicating the current version for each study. This is often provided in a briefing document to BIMO.
Subject Level Data Line Listings By Clinical Site
This section deals with specific patients in each trial, so the level of detail is substantially greater. The FDA points out that certain sites and investigators may be involved in multiple studies. When this is the case, treat each study as a separate entity and organize information accordingly.
Subject-level data line listings, by clinical site, should include consented subjects, treatment assignment, discontinuations, study population, inclusion and exclusion criteria, adverse events, protocol deviations, efficacy endpoints, concomitant medications, and safety monitoring. Appendix 2 illustrates four approaches to organizing and structuring the data, all of which are acceptable. Figure 1 illustrates one such structure.
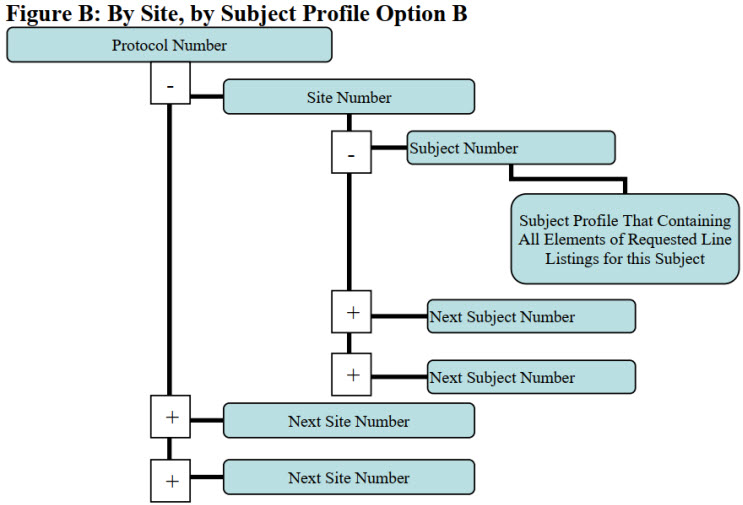
Figure 1: By Site, by Subject Profile Option B. Figure B of the technical document.
All consented subjects should be documented, whether they actually took part in the study or not. The reason for non-enrollment should be described. Patients’ assignments to treatment arms should be described and if they were wrongly medicated, that should be described. Subjects that did not complete treatment should be included with a reason for the discontinuation. The study population of each patient should be identified, and if they did not meet the inclusion/exclusion criteria, that should be indicated. These criteria should be clearly documented along with whether each patient met the criteria or not. All adverse events associated with each patient should be clearly documented. Every protocol deviation should be described with an explanation of the impact on the study. For each patient, a clear statement of whether they met the endpoints should be included. Concomitant medications that each patient took should be described with details of stop and start, dosing, etc. Results of laboratory testing should also be included.
Summary Level Clinical Site Data Set
A single summary-level clinical site data set that contains data from all major (i.e., pivotal) studies used to support safety and efficacy in the application, including studies with different treatment indications, should be provided. For each major (i.e., pivotal) study used to support safety and efficacy, data by clinical site and treatment arm for the safety population (SAFPOP) and primary efficacy population (EFFPOP) should be provided. Again, if a site or investigator was involved in multiple studies, each study should be separately prepared.
For each study and investigator site, it is critical to submit the following variables associated with efficacy with their specific name. Some of the variables that are important include:
- Total number of patients at each site in each arm of the safety population
- Total number of patients at each site in each arm of the primary efficacy population
- The summary statistic of the primary efficacy endpoint at each site for each of the cohorts. How these values were calculated should be described.
- A simple description of the primary endpoint should be described.
- A description of each arm of the trial
- Some endpoints are determined at the end of the study or a predetermined time, while others are time to endpoint. These two should be differentiated. If time to endpoint is not used, that should be left blank.
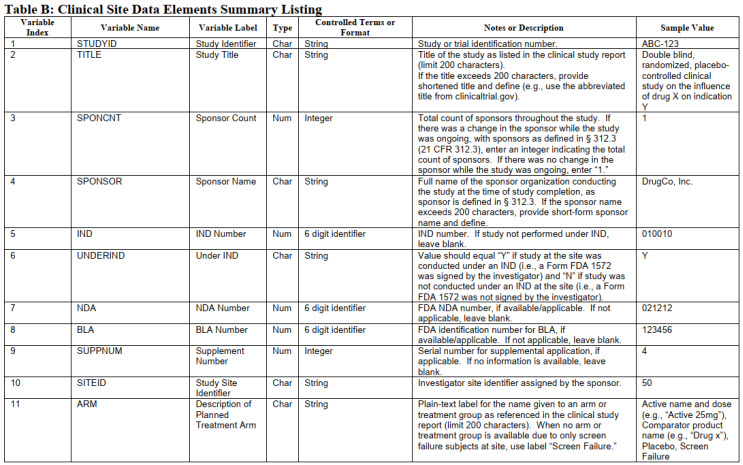
Much more detail on the nomenclature for each of these parameters is described in the technical guide. In addition, it describes the nomenclature of a variety of endpoints commonly used.
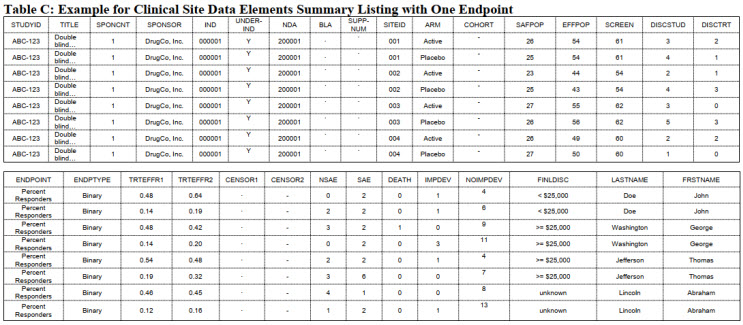
This huge swath of data must be formatted in a consistent manner. Tables 2 and 3 illustrate some sample entries. These are taken from Appendix 3 and 4 and do not include all the information from the technical document. Consult the document for complete details.
Table 2: Clinical Site Data Elements Summary Listing. Part of Table B of technical document.
Table 3: Hypothetical data set from a study. Part of Table C of technical document.
Submitting BIMO Clinical Data In The eCTD Format
These summaries and files will be incorporated into Module 5 in the appropriate sections. The actual construction of each file with hyperlinks is required. These must be positioned in the correct folder with prescribed titles that are included in FDA guidance documents on submission of NDAs and BLAs via the portal. The constructed BIMO study tagging file is placed in the eCTD module 5.3.5.4 “Other Study Reports and related information” section with the study identifier “BIMO.” This guide gives good descriptions of the file names and content.
Another important element to consider is the folder structure. This cannot be downplayed. As an author of sections of submissions during my career, the actual folder structure was always handled by the regulatory affairs department. Larger companies will perform this in-house while smaller companies will employ regulatory companies that specialize in constructing the “correct” file substructure for submission. Doing it yourself is not for the faint hearted.
The document describes the structure required. Figures 2 and 3 illustrate some examples.
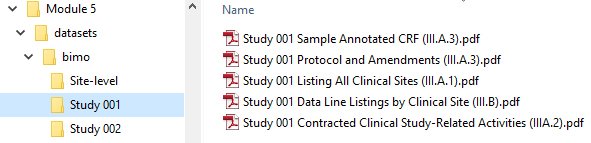
Figure 2: Place Clinical Study-Level Information and Subject-Level Line Listings by Clinical Site in the M5 Folder. Taken from technical document.
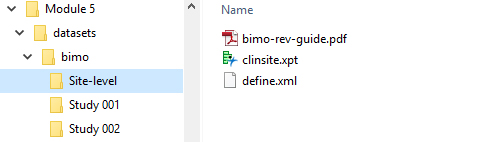
Figure 3: Place the Site-Level Data set Define File and BIMO Data Reviewer’s Guide in the M5 Folder. Taken from technical document.
Guidance is also provided on file format, leaf titles, and submission strategies. You should also read the latest FDA guidances.6,7
References
- BIORESEARCH MONITORING TECHNICAL CONFORMANCE GUIDE: Technical Specifications Document FDA September 2024.
- ICH M4 Common Technical Document 2000 www.ich.org
- ICH M2 Electronic Standards in the transfer of Regulatory Information 2010 www.ich.org
- ICH M8 Electronic Common Technical Document (eCTD) 2008 and 2015 www.ich.org
- Providing Regulatory Submissions in Electronic Format – Certain Human Pharmaceutical Product Applications and Related Submissions Using the eCTD Specifications (February 2020). https://www.fda.gov/regulatory-information/search-fda-guidance-documents
- Study Data Standards Resources https://www.fda.gov/industry/fda-resources-data-standards/study-data-standards-resources
- Transmitting Electronic Submissions Using eCTD Specifications: Technical Specifications Document http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/FormsSubmission
Requirements/ElectronicSubmissions/UCM163567.pdf
About The Author:
 Peter H. Calcott, D.Phil., is president and CEO of Calcott Consulting LLC, which delivers solutions to pharmaceutical and biotechnology companies in the areas of corporate strategy, supply chain, quality, clinical development, regulatory affairs, corporate compliance, and enterprise e-solutions. He has also served as an expert witness. He also teaches at the University of California, Berkeley in the biotechnology and pharmaceutics postgraduate programs. Previously, he was executive VP at PDL BioPharma, chief quality officer at Chiron and Immunex Corporations, and director of quality assurance for SmithKline Beecham and for Bayer. He has also held positions in R&D, regulatory affairs, process development, and manufacturing at other major pharmaceutical companies. He has successfully licensed products in the biologics, drugs, and device sectors on all six continents. Calcott holds a doctorate in microbial physiology and biochemistry from the University of Sussex in England. He has been a consultant for more than 20 years to government, industry, and academia.
Peter H. Calcott, D.Phil., is president and CEO of Calcott Consulting LLC, which delivers solutions to pharmaceutical and biotechnology companies in the areas of corporate strategy, supply chain, quality, clinical development, regulatory affairs, corporate compliance, and enterprise e-solutions. He has also served as an expert witness. He also teaches at the University of California, Berkeley in the biotechnology and pharmaceutics postgraduate programs. Previously, he was executive VP at PDL BioPharma, chief quality officer at Chiron and Immunex Corporations, and director of quality assurance for SmithKline Beecham and for Bayer. He has also held positions in R&D, regulatory affairs, process development, and manufacturing at other major pharmaceutical companies. He has successfully licensed products in the biologics, drugs, and device sectors on all six continents. Calcott holds a doctorate in microbial physiology and biochemistry from the University of Sussex in England. He has been a consultant for more than 20 years to government, industry, and academia.