Getting A Handle On Clinical Trial Costs
By Amit Pratap Singh Rathore, principal analyst, Beroe Inc.
Recently, clinical trials have become very complex affairs, especially for small and medium biotech companies. There are many factors behind this, including, but not limited to, increased costs and regulatory requirements. According to Tomasz Sablinski, CEO at Transparency Life Sciences, the cost of clinical trials increased by around 100 percent from 2008 to 2019. A study published in JAMA Internal Medicine in 2018 found that out of 138 pivotal trials assessing 59 new therapies that received FDA approval between 2015 and 2016, there was a more than 100-fold difference in the costs of clinical trials. It also found that a central cluster of trials had estimated costs of $12.2 million to $33.1 million. In the past five years, the industry has witnessed a lot of consolidation and the birth of giant CROs. The rise of these giant CROs has changed the market landscape significantly and requires pharmaceutical companies to change the way they deal with CROs. The sponsor companies must be more vigilant and thorough in their planning and oversight of trials conducted by vendors. A proper estimation of clinical trial cost could help both large and small pharmaceutical companies in planning their outsourcing of trials. Hence, it is imperative that procurement teams develop a clear understanding of the various cost drivers and parameters of the cost of a clinical trial. This article discusses the major cost drivers and tries to present a qualitative picture of trial cost components.
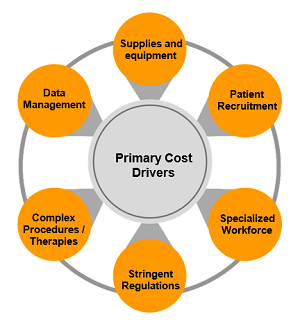
Clinical Trial Cost Drivers
Numerous factors affect the cost of a clinical trial. The major ones are:
- Increased costs of clinical supplies and equipment
- Extended timelines of clinical trials
- Increased regulations, particularly at the clinical and CMC levels
- Monitoring complexities
- Patient recruitment intricacies
- Workforce competence
- Data collection and synergy complexities
Clinical Trial Cost Components
The cost of a clinical trial can be calculated in terms of the cost incurred for analyzing each patient at each site during the study. To estimate the overall cost, many parameters must be considered. The following table captures a sample of the various cost components involved in a clinical trial.
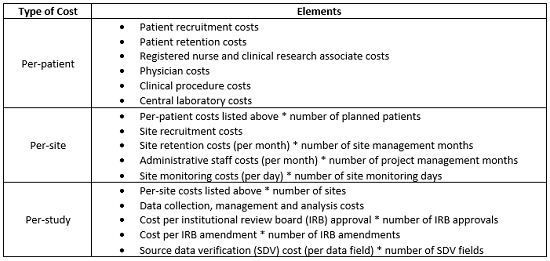
Procurement teams must adopt a detailed approach while estimating the cost per components. Involvement of stakeholders from all departments is vital. Due to the complex regulatory environment, it is easily possible to go wrong on certain cost parameters if operational teams are not consulted. For example, estimating the cost of data management is very tricky. Currently, regulatory authorities are focused on integrity of data. Each data point costs a lot and may or may not be influenced by following factors:
- The patient recruitment process often requires patients to complete four or more questionnaires. This may or may not lead to any meaningful interpretation, but it does elongate the patient recruitment process.
- During clinical trials, the cost of tests such as MRIs is higher (generally three to four times) than the cost of comparable tests during general routine medical visits. This is because university or research organizations offset the grant money for the routine tests or the government subsidizes these tests in hospitals for the general public.
- Sometimes, detailed biomarker analysis samples are sent to different external labs, which attract higher fees.
- Similarly, due to specific procedural requirements, it is possible that physicians perform specialized tests for particular disease indications along with regular physical examinations of patients. This may require more physician time that results in higher costs.
- All the data collected is required to go through stringent data management protocols to ensure data integrity and patient safety. This might require study coordinators to spend more time to fulfill the requirements of GCP guidelines, which further adds to the costs.
Clinical Trial Cost Distribution By Phase
According to the study published in JAMA Internal Medicine in 2018, the costs of trials ranged from $2.1 million for a trial that enrolled four patients to test uridine triacetate for a rare hereditary metabolic disorder to $346.8 million for a noninferiority trial that assessed the impact of a new combination cardiovascular drug for chronic heart failure on hospitalization and cardiovascular mortality. The clinical trials cost a median of $41,117 per patient and $3,562 per patient visit. Clinical trial cost varies with the therapeutic area as well.
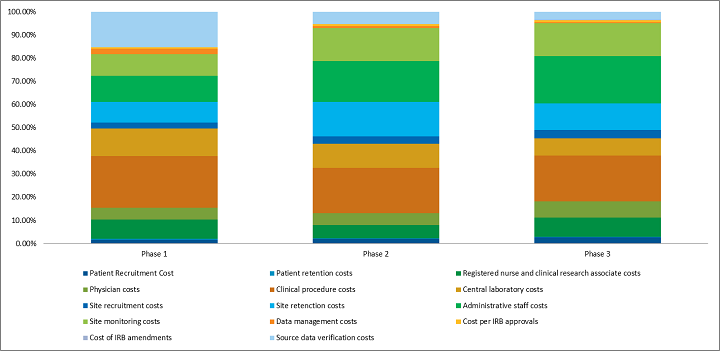
Such data points can help in estimating and benchmarking clinical trial costs. But a cautious approach must be adopted, as these costs could vary according to the particular requirement of drug/therapy/procedure. For example, clinical trial requirements of cell therapies are more stringent and cost-intensive than those of common drug therapies.
How To Select The Most Suitable Partner
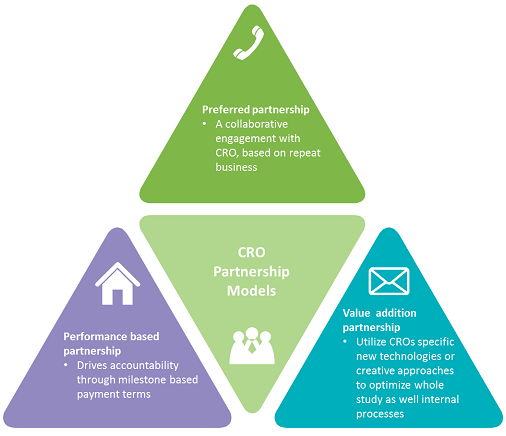
There are basically three models to procure CRO services:
- Preferred vendor model
- Performance-based model
- Value-addition model
A good understanding of the various intricacies involved in the cost of a clinical trial helps procurement and finance teams in budgeting and efficient execution of the whole process. Procurement must understand the criticality of the study and the respective study requirements. There are many service providers in the market, ranging from generalists to specialists. The best approach to select the most suitable CRO starts with understanding study requirements and specificities.
There is a growing concern among sponsors that the large CROs will increasingly focus on the big money from large pharma to the exclusion of small or medium pharmaceutical companies. Hence, the procurement and finance teams must weigh the annual volume of their business against the annual business of CROs they are considering. Technical competency, service commitments, and cost are among the other factors to analyze to find a suitable CRO partner. The selection process must include an in-depth “apples to apples” comparison. It is possible that a CRO may appear cost-effective but in reality lacks efficiency and productivity, which increases the overall cost of the study. One simple way to make an effective comparison is to require different CROs to bid using the same RFP template. An effective analysis must give weight to communication frequency and innovation.
References:
- Moore T, Zhang H, Anderson G, Alexander GC. Estimated costs of pivotal trials for novel therapeutic agents approved the US Food and Drug Administration, 2015-2016 [published online September 24, 2018]. JAMA Intern Med.
- https://www.ncbi.nlm.nih.gov/pubmed/26908540
About The Author:
 Amit Rathore is principal analyst at Beroe Inc. and an Institute for Supply Management (ISM) Certified Professional in Supply Management (CPSM). He is responsible for handling customized procurement assignments related to sourcing, category management, supplier relationship management, technology, risk, and compliance. He has supported Fortune 500 companies in their strategic decisions on sourcing and managing direct and indirect spend categories. You can contact him at amit.rathore@beroe-inc.com or connect with him on LinkedIn.
Amit Rathore is principal analyst at Beroe Inc. and an Institute for Supply Management (ISM) Certified Professional in Supply Management (CPSM). He is responsible for handling customized procurement assignments related to sourcing, category management, supplier relationship management, technology, risk, and compliance. He has supported Fortune 500 companies in their strategic decisions on sourcing and managing direct and indirect spend categories. You can contact him at amit.rathore@beroe-inc.com or connect with him on LinkedIn.
