Gilead Addresses Health Equity In HIV Prevention Challenges By Designing Next-Gen Clinical Trials, Aiming For New Options
By Moupali Das, MD, MPH, HIV Prevention Clinical Development Lead, Gilead Sciences
Steady progress has been made toward global HIV elimination goals, yet persistent disparities in prevention and treatment outcomes remain. Recent data shows that there were 1.5 million new cases of HIV globally in 2021, with significant disparities in vulnerable populations who are often historically underrepresented in HIV clinical trials. Furthermore, fewer than 20% of people who would benefit from pre-exposure prophylaxis (PrEP) are on biomedical intervention for HIV prevention in the U.S. and, globally, less than 10% of the UNAIDS 2025 goal of 10 million are receiving PrEP. These new HIV infections, combined with low real-world uptake of existing PrEP options, demonstrate at least two important insights. First, there is a need to develop more options to provide new choices that may help improve adherence and better fit into the lives of people who would benefit from PrEP (PWBP), possibly bringing more people into HIV prevention care and services. Second, we must be intentional in our innovation, with attention to health equity, as there is a critical need to bring forward clinical strategies that are informed by and designed for the very communities that have these unmet needs.
New Options Needed For Improved Uptake
Guided by innovation in science and in health equity, Gilead designed its Phase 3 PURPOSE trials in HIV prevention research in close collaboration with a diverse range of community advisors who represent populations that are disproportionately affected by HIV. These leaders provide valuable ongoing guidance and support for the trial, including community engagement, participant recruitment strategies, cultural considerations, and appropriate language in outreach material.
The PURPOSE trials study lenacapavir, a potent, first-in-class multi-stage HIV capsid inhibitor that can be administered as a twice-yearly subcutaneous injection. Currently, twice-yearly lenacapavir is being evaluated as a long-acting option in multiple ongoing and planned early and late-stage clinical studies as part of Gilead’s HIV prevention and treatment research program. Notably, lenacapavir is approved in multiple countries for the treatment of people with multi-drug resistant HIV in combination with other antiretroviral(s). Lenacapavir is being studied in the PURPOSE program for the prevention of vaginal (PURPOSE 1, NCT04994509) and rectal (PURPOSE 2, NCT04925752) acquisition of HIV. The use of lenacapavir for HIV prevention is investigational.
PURPOSE 2 is assessing lenacapavir as a potential long-acting drug that may prevent rectal acquisition of HIV in cisgender men who have sex with men, transgender women, transgender men, and gender non-binary individuals who have sex with partners assigned male at birth. PURPOSE 2 is being conducted in the U.S., South Africa, Brazil, Peru, Thailand, and Argentina, where these communities continue to experience disproportionate rates of HIV. The aim is to offer more options and potentially increase the number of individuals gaining access to PrEP care.
A More Ethical Alternative To Placebo Comparator Trials
However, there are several challenges facing the field of HIV prevention clinical trial design. With four currently approved and available options, it is no longer ethical to compare new PrEP options to a placebo. Additionally, it is increasingly infeasible to conduct superiority PrEP trials, as showing superiority essentially requires nonadherence to the comparator. Noninferiority trials, such as DISCOVER (NCT02842086), which compare a new PrEP drug to an active comparator, require increasingly large cohort sizes and long durations. Moreover, noninferiority trials in populations where there is inconsistency in the prior clinical trial results for the comparator are challenging. For instance, in the case of cisgender women, two of the original placebo-controlled trials, FemPrEP and VOICE, did not show efficacy of emtricitabine/tenofovir disoproxil fumarate (FTC/TDF; sold commercially as Truvada®) for PrEP, but the Partners PrEP trial did demonstrate efficacy. This inconsistency precludes the estimation of the noninferiority margin, which requires pooling of the efficacy estimates from the historic trials. However, given that we know that FTC/TDF for PrEP is highly effective in cisgender women from further analyses of the original trials as well as subsequent demonstration projects and RWE, the prior trial results make it infeasible to use FTC/TDF for PrEP as a noninferiority comparator in HIV prevention studies enrolling cisgender women. This is why Gilead was unable to conduct a noninferiority trial comparing emtricitabine/tenofovir alafenamide (FTC/TAF; sold commercially as Descovy®) for PrEP to FTC/TDF for PrEP in cisgender women.
To address this challenge in the PrEP clinical trial space, the Forum for Collaborative Research convened regulatory agencies, intergovernmental groups, academic/nonprofit institutions, civil society/community, drug developers, and diagnostics companies to develop a consensus statement on the design of next-generation PrEP trials. The proposed new trial design suggests using a different method, such as recency assays and the recent HIV infection testing algorithm, to estimate the counterfactual background HIV incidence instead of randomizing participants to placebo, which is unethical. Another case where it is unethical to randomize to placebo is in the development of new options for hormonal contraception. The Pearl Index is used to develop new drugs for pregnancy prevention. One can use the known fertility rate (80/100 person-years) in reproductive-age women as the counterfactual pregnancy incidence. The trial can use a single-arm open-label design, where 100 sexually active women use the new contraceptive. If the incidence of pregnancy declines 80- or 40-fold to only one or two pregnancies, these results strongly suggest high efficacy of the new contraceptive for pregnancy prevention.
This design also can be applied to HIV prevention trials. If we can estimate the counterfactual HIV incidence in a population, then we can observe the incidence on an investigational PrEP drug(s) and compare it to the expected HIV incidence. If there is a notable decline in HIV incidence, this strongly suggests efficacy of the HIV PrEP drug. The beauty of this design is the evaluation of inherent efficacy rather than comparative efficacy. These designs require knowledge of the background incidence of the outcome we are trying to prevent (pregnancy or HIV incidence). The higher the background incidence, the easier it will be to demonstrate a definitive decline on the study product.
How can we determine the counterfactual HIV incidence? HIV epidemiologists and public health professionals have been using the recent HIV infection testing algorithm to estimate HIV incidence in a specific geography or population for several years. One can conduct a cross-sectional survey of a particular population, perform standard HIV testing on positive blood samples, conduct a specialized HIV test called the HIV recency assay, and use the recent infection testing algorithm (RITA) to estimate HIV incidence. Normal serologic HIV tests measure the antibodies to HIV in the blood. A recency assay takes advantage of how our immune system evolves to become better at recognizing HIV to determine if the HIV infection was recent. Initially, in HIV infection, antibodies have low avidity or stickiness to HIV. As the immune system adapts to HIV, antibodies become more avid or stickier to HIV. We can use this relationship to determine if HIV infection is recent by using a simple laboratory assay that measures antibody avidity, if there is low antibody stickiness indicating recent HIV infection, then there will be lower optical density on the recency assay test, which suggests recent HIV infection.
Gilead is applying the aforementioned public health tool to estimate the HIV incidence in a population for the PURPOSE clinical trials. Prior to the standard randomized trial phase, an incidence screening phase is conducted. Individuals who meet general eligibility criteria for the trial(s) are tested for HIV using standard methods. Those who test negative proceed to further eligibility screening and randomization to study products, while those with a positive diagnosis are referred to HIV care. Samples that test positive for HIV using the standard test are then subjected to a recency assay. If the recency results and HIV viral load results are consistent with recent HIV infection, that data is used to estimate the counterfactual HIV incidence. This estimate is used to determine the HIV incidence that would occur if people were not on PrEP, which enables the evaluation of the inherent efficacy of new PrEP drugs.
By using this method, we can estimate the counterfactual background HIV incidence in individuals who are eligible for the trial but not taking PrEP. This enables us to compare the HIV incidence in those who are randomized to the study drug with a population that is as similar to them as possible, without randomly assigning them to a placebo in an unethical manner.
The primary endpoint of PURPOSE 2 evaluates the inherent efficacy of lenacapavir by comparing the HIV incidence on lenacapavir with the estimated counterfactual background HIV incidence using recency assays and the RITA in the screened population. PURPOSE 1, which was described in a “Clinical Leader” article published in 2021, has two primary endpoints and is evaluating both the inherent efficacy of lenacapavir as well as FTC/TAF for PrEP in cisgender adolescent girls and young women. Both PURPOSE trials are actively enrolling participants, and results are expected in 2025. Gilead presented details about this innovative trial design and its implementation in two invited presentations at the 12th International AIDS Society Conference on HIV Science, and overviews are available here and here.
Beyond The Science, Closer To People Who Would Benefit From PrEP
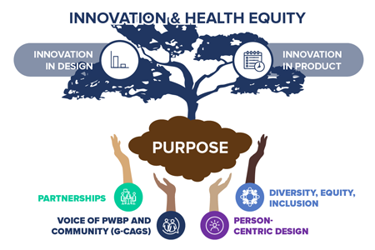
Gilead recognizes the significance of being intentional with our innovation to avoid exacerbating existing health disparities. Therefore, we applied our four foundational pillars of health equity to the PURPOSE program.
- Working With Key Stakeholders
- At the earliest stages of planning for the PURPOSE 2 trial, a regular and growing number of stakeholders from the U.S., South Africa, Brazil, Peru, Argentina, and Thailand (soon to include Mexico and Vietnam) — where unmet HIV prevention needs are the greatest — were consulted.
- These stakeholders offered diverse experiences and perspectives, representing community groups, academic HIV researchers and scientists, government officials, and policymakers.
- The consultations with key stakeholders informed relevant parties about the novel design, sought feedback about how best to educate key decision-makers about the novel design, and raised awareness and scientific clinical trial design literacy. Feedback informed how we described the design in our protocol, drafted informed consent forms, and shaped communications with future potential participants, community advocates, site investigators, and site staff.
- We meet with our investigators and site staff monthly to continue this process of real-time engagement and collaboration.
- The Voice Of PWBP And Community
- Equipped with the momentum and support of stakeholder partnerships, we have established Global Community Advisory and Accountability Groups (G-CAGs) for each PURPOSE study. The G-CAGs have representation from historically underrepresented and disproportionately affected populations who would most benefit from PrEP.
- We followed good participatory practices guidelines to solicit feedback on an ongoing basis from the groups on the trial protocol, communication strategies, recruitment and retention strategies during the planning stage, and implementation.
- We meet virtually with our G-CAGs every other month and have face-to-face meetings throughout the year at conferences and in dedicated G-CAG meetings. We also hold joint meetings that bring together our investigators and G-CAG members to ensure the most robust representation of the voice of people who would benefit from PrEP in the clinical trial process.
- Furthermore, we seek input from individuals who may not be part of the G-CAGs but represent affected communities that would benefit most from additional potential PrEP options. This includes local people who have been affected by HIV, community advocates, individuals working or volunteering in local clinics or community centers, government officials, public health experts, and public health officials.
- Person-Centric Design
- Insightful feedback from stakeholders and G-CAGs underscored the vital importance of appropriately providing support to reduce barriers to trial enrollment and retention, such as food, transportation, educational/vocational, and empowerment initiatives.
- This involved selecting trial sites with established expertise in engaging vulnerable populations, particularly those with experience in gender-affirming care, to facilitate the inclusion of gender-diverse individuals.
- Furthermore, site-specific trainings on anti-racism, gender diversity, and cultural humility were developed, implemented, and made mandatory for Gilead staff, PIs, sites, vendors, and CROs. The trainings were created in collaboration with internal experts from disproportionately affected communities and in consultation with external collaborators.
- Diversity, Equity, And Inclusion
- We have established global goals for the inclusion of historically excluded and disproportionately affected populations in PURPOSE 2. Specifically, in the U.S., we have set goals for the inclusion of 50% Black and 20% Hispanic/LatinX cisgender men who have sex with men. We have also set a goal of 20% transgender women globally.
- To implement these goals, we have set site-specific enrollment goals for race, ethnicity, and gender based on the local epidemiology of new HIV infections and the general demographics of the local area. Site investigators and the Gilead study team agreed upon these goals prior to the initiation of the study. The recruitment numbers are reviewed internally weekly and with the site investigators monthly to track progress on achieving the study-wide goals.
- PURPOSE 2 intentionally includes transgender men and gender nonbinary individuals for the first time in any Phase 3 PrEP trial. Due to the paucity of data, specific recruitment goals were not set for these populations.
- Based on feedback from our partners and the G-CAGs, we are conducting an evaluation of lenacapavir exposure in persons on gender-affirming hormone therapy to support the future potential use of the investigational product in gender-diverse populations, provided efficacy is demonstrated. Although we do not anticipate any interactions between lenacapavir and gender-affirming hormone therapy based on our understanding of their pharmacokinetics, we are conducting a substudy to gather clinical data from the PURPOSE 2 trial.
- Both PURPOSE 1 and 2 are including adolescents for the first time in the pivotal PrEP trials.
- Lastly, as previously described, we are including pregnant and lactating people in PURPOSE 1 and conducting lenacapavir exposure assessments during pregnancy, postpartum, in infants, and in breastmilk to support the potential use of this investigational product in adolescent girls and young women, should efficacy be demonstrated.
- In June 2022, we shared our experience with these four pillars of health equity and specifically highlighted our diversity and inclusion goals in our first-ever publication on the PURPOSE 2 trial titled Proactive strategies to optimize engagement of Black, Hispanic/Latinx, transgender, and nonbinary individuals in a trial of a novel agent for HIV pre-exposure prophylaxis. The research article was co-authored by our investigators and the community advisors on the G-CAGs.
Taken together, we hope that the dual commitments to innovation in the science, including clinical trial design, and innovation in the four pillars of health equity in the PURPOSE studies will help advance the collective goal of developing new options for HIV prevention. By working collaboratively with key stakeholders to develop the novel counterfactual design, we are able to conduct these two critical HIV prevention trials with a goal to develop new options for PrEP that are widely accepted, have high uptake and persistence, and ultimately help end the HIV epidemic for everyone, everywhere.
The use of lenacapavir for HIV prevention is investigational and the safety and efficacy of lenacapavir for this use have not been established. Gilead is studying the safety and efficacy of lenacapavir for HIV prevention in multiple ongoing clinical studies. The use of FTC/TAF in cisgender adolescent girls and young women who are at risk of acquiring HIV through vaginal sex is investigational, and the safety and efficacy of FTC/TAF for this use have not been established.
About The Author:
 Moupali Das, MD, MPH, is an executive director, HIV clinical development, in the virology therapeutic area at Gilead Sciences, where she leads the PrEP clinical drug development program. Moupali is an infectious disease specialist with additional training in HIV prevention, and her MPH is in epidemiology. Moupali has led high-performing teams in academic medicine, public health, implementation science, and cross-functionally in drug development. She has helped develop, implement, and evaluate how to better test, link to care, increase virologic suppression, and improve quality of life for people with HIV and to prevent HIV in those who may benefit from PrEP.
Moupali Das, MD, MPH, is an executive director, HIV clinical development, in the virology therapeutic area at Gilead Sciences, where she leads the PrEP clinical drug development program. Moupali is an infectious disease specialist with additional training in HIV prevention, and her MPH is in epidemiology. Moupali has led high-performing teams in academic medicine, public health, implementation science, and cross-functionally in drug development. She has helped develop, implement, and evaluate how to better test, link to care, increase virologic suppression, and improve quality of life for people with HIV and to prevent HIV in those who may benefit from PrEP.
