Global AI In Clinical Trials: Market Trends & Current Partnerships
By Mathini Ilancheran, senior delivery lead - research, R&D, Beroe Inc.

Updated: 12:45pm, February 24, 2025
Currently, the pharmaceutical industry has adopted a conservative approach toward large-scale AI adoption due to the caution needed when dealing with human life, regulatory uncertainties, and substantial capital requirements. AI-based models have been used to complement traditional experimental methods, ensuring the safety and efficacy of drugs. AI integration holds the potential to accelerate the creation of novel treatments, reduce costs, and improve patient outcomes. However, to fully harness AI's potential in pharmaceutical research and development, challenges related to data accessibility, algorithm interpretability, and regulatory frameworks must be addressed.
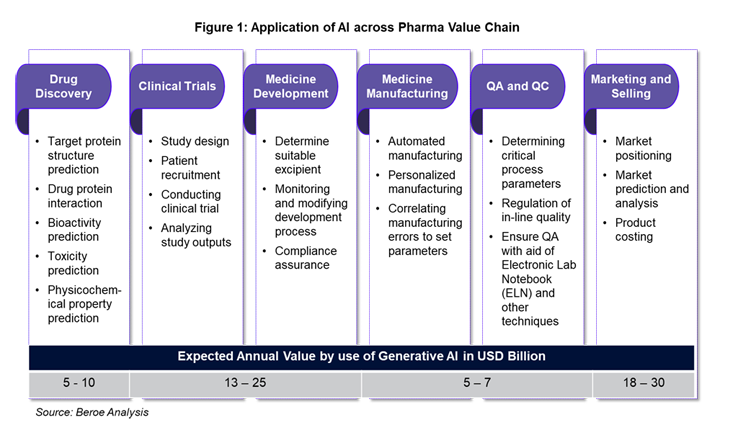
Trends Of AI In Clinical Drug Development
Market Growth And Potential
The global AI in clinical trials market is valued at approximately $2.7 billion in 2025. It is expected to grow at a compound annual growth rate (CAGR) of 24 to 28%, reaching $8.5 billion by 2030 (Figure 2)1.
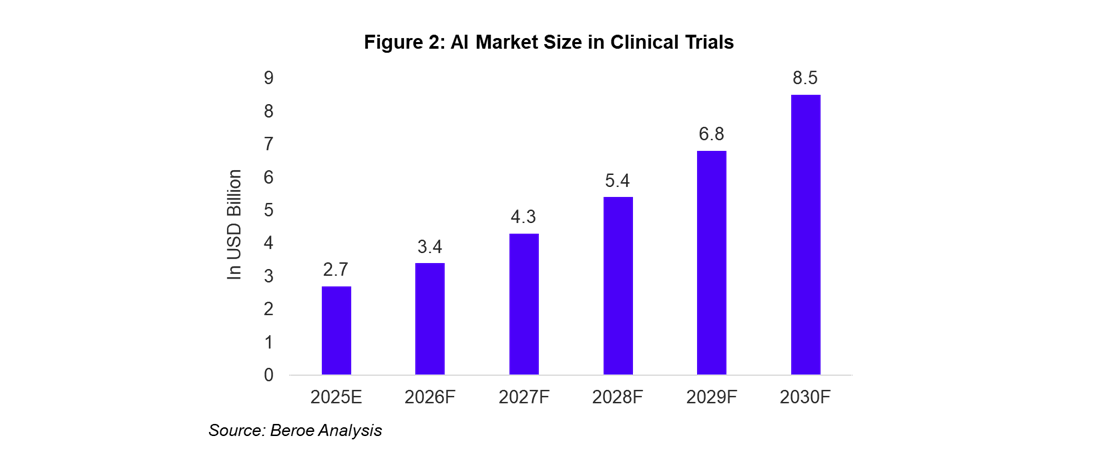
By 2030, AI is expected to be integrated into 60–70% of clinical trials, saving the pharmaceutical industry $20–30 billion annually through cost efficiencies and faster trial timelines. Niche applications, such as AI for rare disease trials, are likely to see even greater adoption and growth.2
Application And Returns
AI applications in clinical research are primarily concentrated in oncology, especially in patient recruitment — one of the most challenging aspects of clinical trials. AI enhances efficiencies by reducing sample sizes, improving enrollment rates, and enabling faster, more optimized adaptive trials. As of December 2022, the leading 800 AI-driven pharmaceutical companies have collectively received approximately over $59.3 billion in funding, reflecting a significant increase from the $37 billion reported in the previous year 3. This surge in investment underscores AI's transformative potential in reshaping both clinical trials and drug discovery, promising groundbreaking therapies and substantial economic benefits for the pharmaceutical industry over the next decade.
Clinical trials using AI technologies have the potential to significantly enhance outcomes in several key areas. A few examples include:
- Approval at a Lower Cost — According to IQVIA, a clinical research site leveraged AI- and ML-powered technology to streamline feasibility survey completions, achieving a 90% reduction in the time required.
- Faster Time to Market — A biopharmaceutical company shortened trial durations by 15% to 30% by substituting the traditional endpoint for a rare disease with more frequently occurring endpoints or those measurable through blood tests4.
- Improved Patient Outcomes — ML-driven prediction models have enabled a 15% to 25% decrease in cancer mortality rates in multiple clinical trials5.
- Cancer Drug Trials Optimization — Leveraging AI in partnership with Immunai, AstraZeneca reduced trial durations by up to 25%, optimizing dose selection and improving biomarker identification in cancer drug trials11.
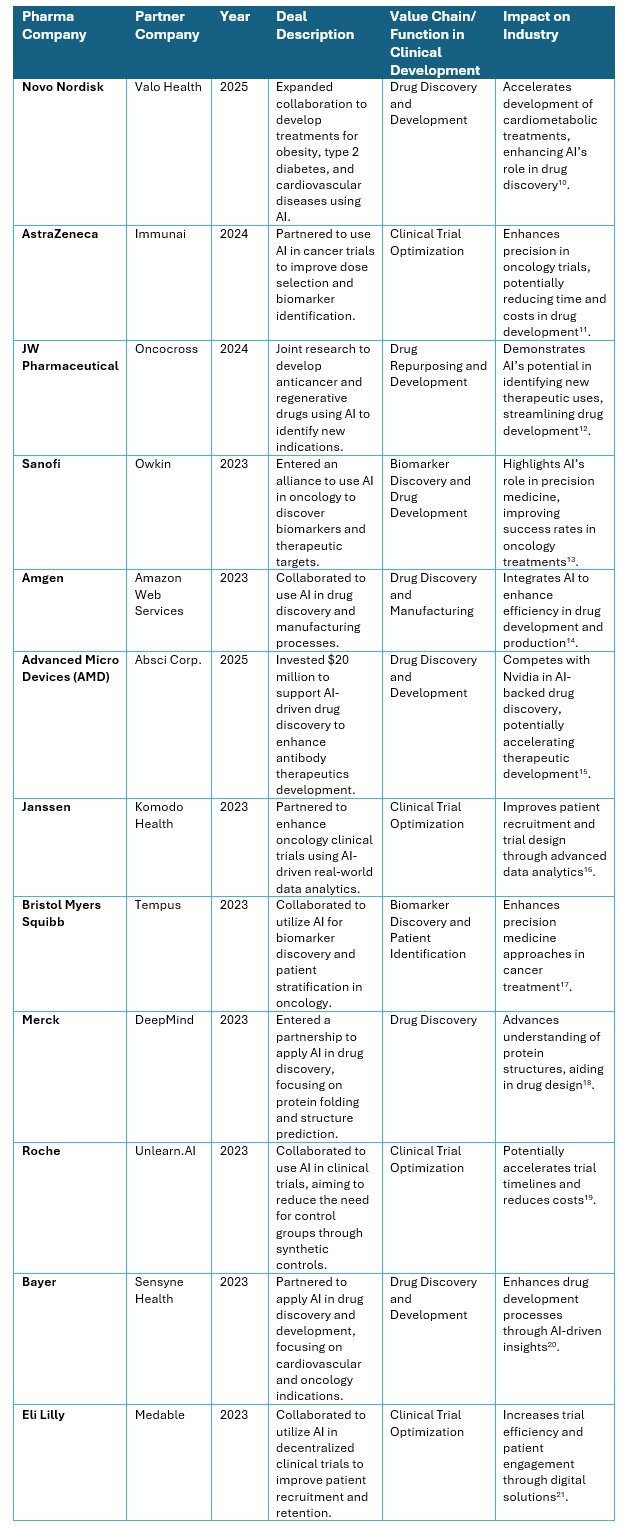
AI-Driven Clinical Trials Partnerships
From 2022 to 2025, pharmaceutical companies have increasingly partnered with technology firms to apply AI in clinical research, drug development, and manufacturing. These collaborations aim to optimize clinical trial design, improve patient recruitment and retention, enhance real-world evidence analytics, and streamline manufacturing processes. Investments in these areas are estimated to range between $2 billion and $4 billion USD, reflecting the sector’s emphasis on integrating AI to reduce costs, accelerate timelines, and improve efficiency6,7.
The following table outlines key partnerships established between pharmaceutical and technology companies from 2023 to the present.
Conclusion
The increasing adoption of AI, with a 30% rise in partnerships from 2022 to 2024, underscores the industry’s recognition of AI’s transformative potential. AI technologies are enabling real-world data integration, improving trial design, and fostering decentralized trials, paving the way for more accessible and patient-centric drug development processes. Furthermore, advancements in AI-driven manufacturing are addressing scalability and production efficiency, critical for meeting growing global healthcare demands8,9.
As AI continues to mature, the pharmaceutical industry’s embrace of this technology reflects a commitment to innovation and efficiency, ultimately promising faster delivery of safer, more effective therapies to patients worldwide. The trends and partnerships highlighted not only mark a turning point in clinical research but also establish a solid foundation for the future of precision medicine and value-based healthcare.
References:
- Research and Markets, “www.globenewswire.com,” www.globenewswire.com, 6 January 2025. [Online]. Available: https://www.globenewswire.com/news-release/2025/01/06/3004773/28124/en/AI-in-Clinical-Trials-Market-Research-2024-2030-with-In-depth-Assessment-of-Market-Shares-Growth-Strategies-and-Service-Offerings-of-Leading-Players.html.
- Accenture, "AI: Healthcare’s New Nervous System," 2021. [Online].
- N. R. Narain, “A New Era Of Drug Discovery With Biology-Driven AI,” Forbes, 14 February 2024. [Online].
- C. Anagnostopoulos, D. Champagne, T. Devenyns, A. Devereson and H. Tarkkila, “How artificial intelligence can power clinical development,” McKinsey & Company, 22 November 2023.
- A. S, B. D and C. G, “Artificial Intelligence Applied to clinical trials: opportunities and challenges,” Health Technol Springer, 28 February 2023.
- FierceBiotech, "Big Pharma's Bet on AI-Driven Clinical Trials and Development," 2024.
- Pharmaceutical Technology, "AI Adoption in Pharma's Clinical Operations," 2023.
- MarketWatch, "AI in Clinical Drug Development and Manufacturing," 2024.
- EvolveETFs, "AI Partnerships Revolutionizing Healthcare Sector Investments," 2023.
- Novo Nordisk and Valo Health, "Expanded collaboration to develop treatments for obesity, type 2 diabetes, and cardiovascular diseases using AI," Press Release, 2025.
- C. Hale, “AstraZeneca extends AI immuno-oncology R&D pact with Immunai,” FIERCE Biotech, 26 September 2024 . [Online]. Available: AstraZeneca extends AI immuno-oncology R&D pact with Immunai. [Accessed 15 February 2025].
- JW Pharmaceutical and Oncocross, "Joint research to develop anticancer and regenerative drugs using AI to identify new indications," JW Pharmaceutical, 2024.
- Sanofi and Owkin, "Entered an alliance to use AI in oncology for discovering biomarkers and therapeutic targets," Sanofi, 2023.
- Amgen and Amazon Web Services, "Collaborated to use AI in drug discovery and manufacturing processes," Amgen, 2023.
- Advanced Micro Devices (AMD) and Absci Corp., "Invested $20 million to support AI-driven drug discovery, aiming to enhance antibody therapeutics development," Press Release, 2025.
- Janssen and Komodo Health, "Partnered to enhance oncology clinical trials using AI-driven real-world data analytics," GlobalData, 2023.
- Bristol Myers Squibb and Tempus, "Collaborated to utilize AI for biomarker discovery and patient stratification in oncology," PharmaTimes, 2023.
- Merck and DeepMind, "Entered a partnership to apply AI in drug discovery, focusing on protein folding and structure prediction," DeepMind, 2023.
- Roche and Unlearn.AI, "Collaborated to use AI in clinical trials, aiming to reduce the need for control groups through synthetic controls," Roche Press Release, 2023.
- Bayer and Sensyne Health, "Partnered to apply AI in drug discovery and development, focusing on cardiovascular and oncology indications," Bayer News, 2023.
- Eli Lilly and Medable, "Collaborated to utilize AI in decentralized clinical trials, aiming to improve patient recruitment and retention," Medable, 2023.
About The Author:
 Mathini Ilancheran is an accomplished expert in market intelligence and industry analysis, specializing in delivering strategic insights that help Fortune 500 companies make informed decisions. With a focus on global pharma, biotech, and medical devices, she brings deep expertise in value chain analysis, industry and technology trends, competitive intelligence, and strategy. A prolific thought leader, Mathini has authored 35+ publications on R&D outsourcing, offering actionable perspectives that guide global enterprises in optimizing outsourcing practices, category management, and long-term planning.
Mathini Ilancheran is an accomplished expert in market intelligence and industry analysis, specializing in delivering strategic insights that help Fortune 500 companies make informed decisions. With a focus on global pharma, biotech, and medical devices, she brings deep expertise in value chain analysis, industry and technology trends, competitive intelligence, and strategy. A prolific thought leader, Mathini has authored 35+ publications on R&D outsourcing, offering actionable perspectives that guide global enterprises in optimizing outsourcing practices, category management, and long-term planning.
