How Clinical Trials Execs Adapted To The Pandemic's Digital Demands
By Stephen Kemler, RSM US LLP

As the pandemic started causing major disruptions last spring, Medidata began publishing analysis on its impact on clinical trials. At the worst point, year-on-year enrollment of new patients globally was down 59% in April 2020. By August of 2020 (the last update published), the year-on-year impact was down only 20%.
In early 2020, regulatory agencies paused clinical trials and healthcare systems delayed elective procedures. This was compounded by FDA delays for domestic inspections and widescale halts to foreign inspections. The most obvious result to expect would have been a substantial drop in trial starts and drug approvals. However, by adopting changes like virtual work and new trial technologies, the life sciences industry was able to avoid this fate.
The first step for CROs and biopharma companies was to return their teams to work, even if offices were closed. This required broad adoption of video conferencing, new collaboration tools, and back-office systems to eliminate paper invoices, checks, and signatures. Although CROs and biopharma companies shifted quickly to virtual work themselves, the adoption of new technologies for trials didn’t seem to follow as quickly. A review of journal and news articles did not show a conclusive trend toward adopting new technologies outside of the patient visit and telehealth space. Given the new challenges that are posed in trial management and patient recruitment with distributed and virtual trials, broader adoption of technology to manage these areas is likely as this transition away from traditional site-based trials becomes permanent.
In 2019, there were 1,079 Phase 3 interventional trials started as new therapy investigations according to ClinicalTrials.gov. In 2020, that number was basically flat at 1,061 starts. This reflects both CROs’ and biopharma companies’ ability to adapt to the new environment while successfully addressing the influx of trials related to vaccines and treatments for COVID-19. This positive reaction was a strong indication that the industry’s adaptations and resiliency efforts worked and provided a good framework for addressing future trial disruptions. CROs and biopharmas are now able to reflect and expand on these results and implications in our post-pandemic world.
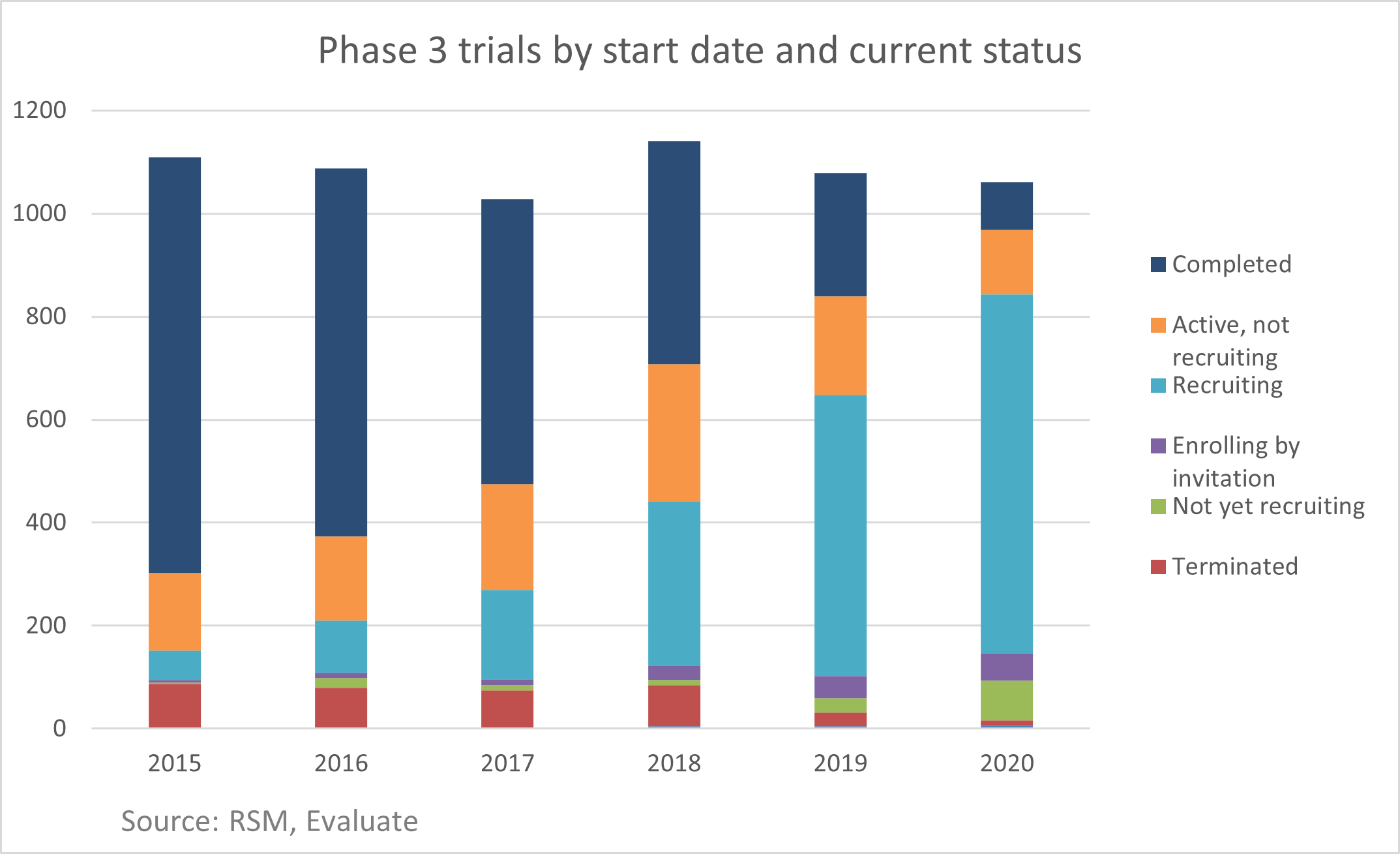
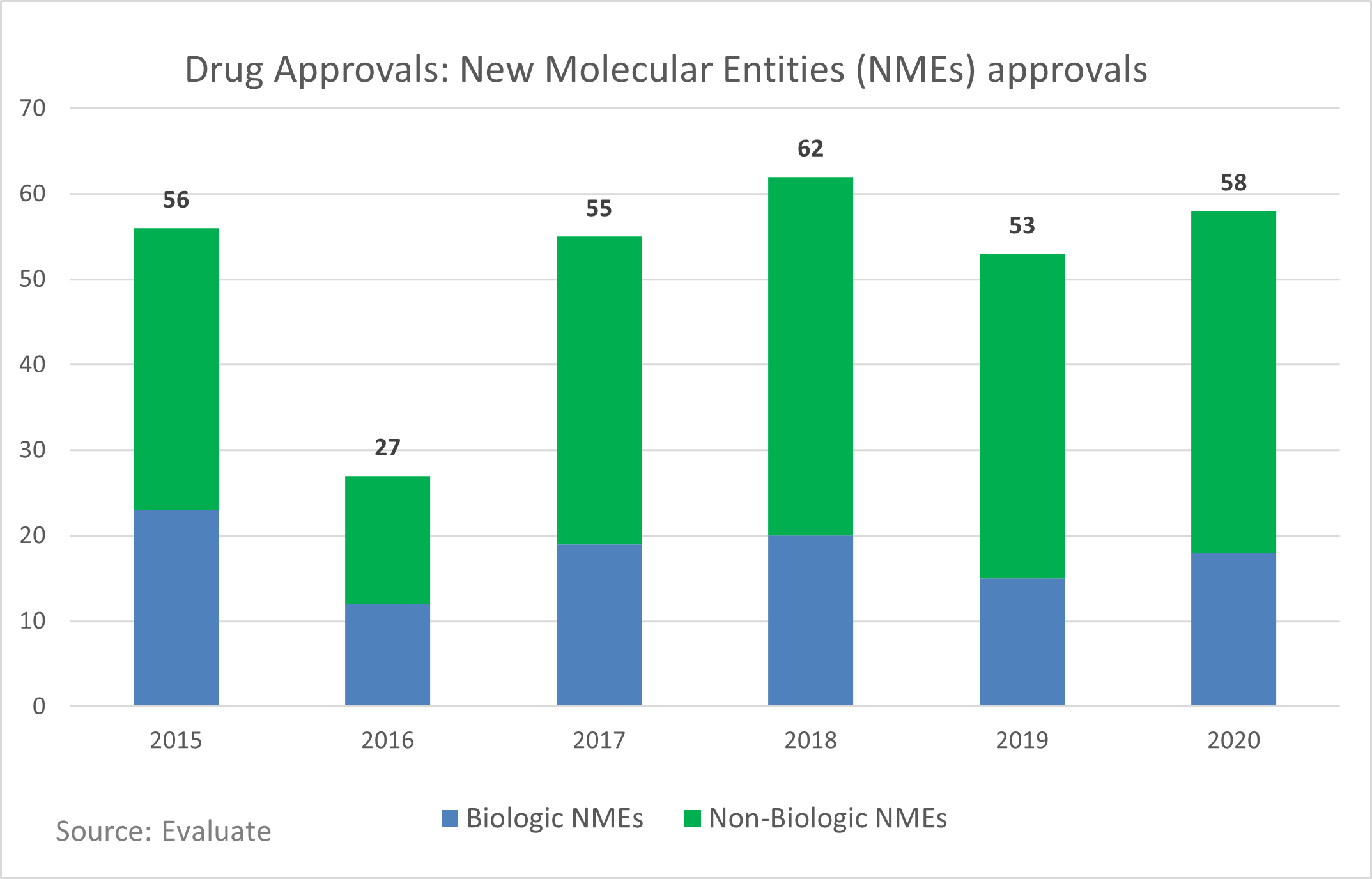
The fact that drug approvals remained on pace and there wasn’t a substantial shift in the types of drugs approved reflects the hard work and difficult choices CROs and biopharma companies made to restart their trials and keep their pipelines moving forward.
As vaccination rates in the United States climb, public health restrictions are relaxed, and life returns to some sense of normalcy, it is important to look back at the impact of 2020 on the life sciences industry, clinical trials, contract research organizations (CROs), and biopharma companies and consider what changes should be permanent. The following questions could provide some critical introspection for CROs and biopharma companies, especially as they consider future steps toward long-standing change or a return to a previous state of operation.
What Can You Do To Make Trial Participation As Easy As Possible For Patients?
While adoption of digital tools for clinical trials remained relatively steady, the way study sites and patients interacted became much more reliant on technology. According to research by ERT, in May 2020, 71% of study sponsors reported adopting telehealth in their trials. This mirrors a trend reported by J.D. Power that prior to 2020, only 9% of patients used telehealth in any form; this jumped to 36% in 2020. Telehealth visits allowed 76% of patients to remove transportation as a barrier to care and 65% to avoid having to take time away from work for doctor visits according to a study from the COVID-19 Healthcare Coalition.
One of the reasons that the healthcare and trial community was able to pivot so quickly to telehealth use is that the United States government took significant steps to enable telehealth. During the period when the country was under an active public health emergency declaration, the Department of Health and Human Services waived certain HIPAA requirements to allow the use of FaceTime, Zoom, and other widely available tools in a clinical setting. Additionally, CMS and many payers adjusted reimbursement policies so that telehealth could be reimbursed at parity compared to in-person visits. However, neither of these changes is guaranteed to be permanent after the pandemic.
Removing barriers to trial participation is important for numerous reasons. For example, clinical trials consistently miss patient recruitment goals. According to a study published in the Journal of Clinical Oncology, only 51% of patients agreed to participate in a trial when presented with the option. The drivers for patients declining participation included both a desire for other treatment and, for 13% of these patients, distance to the cancer center. Virtual trials and telehealth visits could remove this reason potential patients might cite for declining to participate in a trial.
As CROs and biopharmas design the next generation of trials, they will need to actively include remote patient visits, direct-to-patient drug delivery, and at-home monitoring. It is also critical to monitor the direction of the rest of the healthcare field. If sites move primarily back to in-person care, then those conducting trials will need to determine if they can work with traditional sites to include telehealth for trial participants or whether to take the more drastic approach of designing truly virtual trials that focus less on traditional sites and leverage new models focused on ease of access and participation by patients.
How Can You Ensure That Trials Are Inclusive And Match Population Demographics?
If the goal is for trial populations to match patient populations, this cannot be left to chance and requires intentional consideration during the design of site and patient recruitment plans. Virtual trials, direct-to-patient shipments, and telehealth all offer tools to reduce the barriers that prevent people of color and other minorities from participating in trials, including making them more accessible. According to research published in the journal Cancer Control, minority patients are more likely to receive care at under-resourced hospital systems that lack the staff and infrastructure to act as study sites. These patients are also less likely to have adequate medical insurance and thus face financial barriers to participating in trials.
Black Americans are 1.4 times more likely to die of COVID-19 than white Americans according to data collected by the COVID Tracking Project. Despite this stark reality and substantial pressure to ensure that vaccine trials were diverse, neither the Moderna (9.7% of trial participants were Black) or Pfizer/BioNTech (9.8%) trials reached representation in their trials that matched the overall U.S. population (12.3%).
This most likely reflects minorities’ relative lack of access to clinical trials and not any specific failings on the part of these trials. Addressing these shortcomings requires both immediate action while designing new trials and longer-term engagement with specific patient populations. In the short term, diversity and equitable access to trials should be a key point of conversation between sponsors and CROs. This will help ensure these goals are considered at all points of trial design from site selection, patient recruitment plans, and leveraging the right technology to remove barriers to participation. Longer term, it is important for sponsors to engage with these patient communities to build trust and understanding in advance of attempting to recruit for trials.
Will Changes Be Permanent?
So far, 2021 has represented a remarkable shift toward trials that are more equitable, more patient-centric, and more resilient. It would be easy to conclude that these changes are both sufficient and permanent. Unfortunately, there is still more work to be done in cementing these changes and continuing to make trials more inclusive and patient-centric.
In the face of an unfathomable tragedy, the life sciences industry adapted and emerged stronger. Now the industry is left to decide if those changes will last or if trials will go back to the way they were: slower, less diverse, and more difficult for patients. Time will tell, but here’s hoping lessons learned will pave the way for improvements and a step forward rather than a step back.
About The Author:
 Stephen Kemler is a director and life sciences senior analyst at RSM US LLP. He forecasts and communicates economic, business, and technology trends shaping the life sciences industry. He also participates in the Philadelphia Alliance for Capital and Technologies (PACT) medtech series and is involved in multiple regional and national life sciences initiatives. Previously, he provided services for a multinational CRO and also worked at a medical technology company. He can be reached on LinkedIn.
Stephen Kemler is a director and life sciences senior analyst at RSM US LLP. He forecasts and communicates economic, business, and technology trends shaping the life sciences industry. He also participates in the Philadelphia Alliance for Capital and Technologies (PACT) medtech series and is involved in multiple regional and national life sciences initiatives. Previously, he provided services for a multinational CRO and also worked at a medical technology company. He can be reached on LinkedIn.
